Received: 7 March 2023 | Revised: 11 April 2023 | Accepted: 26 April 2023
DOI: 10.1111/prd.12503
REVIEW ARTICLE
Avoiding implant-related complications in medically compromised patients with or without unhealthy lifestyle/Elevated oxidative stress
Gregorio Guabello 1 | Francesco Zuffetti 2 | Andrea Ravidà 3 | Matteo Deflorian 2 |
Giorgio Carta 4,5,6 | Muhammad H. A. Saleh 7 | Matteo Serroni 8 | Bernhard Pommer 9 |
Georg Watzek 9 | Luca Francetti 10,11 | Tiziano Testori 2,7,11,12
- Endocrinology Unit, IRCCS Galeazzi Sant’Ambrogio Hospital, Milan, Italy
- Section of Implant Dentistry and Oral Rehabilitation, IRCCS Galeazzi Sant’Ambrogio Hospital, Dental Clinic, Milan, Italy
- Department of Periodontics and Preventive Dentistry, University of Pittsburgh School of Dental Medicine, Pittsburgh, Pennsylvania, USA
- Argo Academy International Research Bologna, Bologna, Italy
- Private Practice, Bologna, Italy
- Lake Como Institute, Como, Italy
- Department of Periodontics and Oral Medicine, University of Michigan School of Dentistry, Ann Arbor, Michigan, USA
- Department of Innovative Technologies in Medicine & Dentistry, University ‘G. D’Annunzio’, Chieti-Pescara, Italy
- Academy for Oral Implantology, Vienna, Austria
- IRCCS Galeazzi Sant’Ambrogio Hospital, Dental Clinic, Milan, Italy
- Department of Biomedical, Surgical and Dental Sciences, Università degli Studi di Milano, Milan, Italy
- Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, Massachusetts, USA
Correspondence
- Tiziano Testori, Section of Implant Dentistry and Oral Rehabilitation, IRCCS Galeazzi Sant’Ambrogio Hospital, Dental Clinic,Via Cristina Belgioioso 173, Milan, 20157 MI, Italy.
- Email: tiziano.testori@unimi.it
1 | INTRODUCTION
Increased human life expectancy broadens the alternatives for missing teeth and played a role in the widespread use of dental implants and related augmentation procedures for the aging population. Though, many of these patients may have one or more diseases. These systemic conditions may directly lead to surgical complications, compromise implant/bone healing, or influence long-term peri-implant health and its response to biologic nuisances.1 Similar risks may pertain to the medications taken by this population rather than the disease itself.2 Thus, understanding the biological events behind these conditions is indispensable for the implant team.
Offering patients credible expectations regarding intraand postoperative complications and therapeutic prognosis is an ethical and legal obligation. Complications must be explicitly communicated to the patient to render informed consent to every elective surgical intervention. For medically complex patients presenting with synergistic effects of different comorbidities, it is not clear understanding for what preventive measures exactly must be taken.3 Precautions may involve perioperative antibiotic coverage, prolonged and/or submucosal implant healing times, and avoiding bone augmentation surgery.3 The bone and soft tissue response following endosseous implant placement are associated with a cascade of molecular events controlled by wound-healing mediators (cytokines, chemokines, and growth factors) as well as mineral metabolism (hormones and diet) potentially affecting initial osseointegration as well as long-term success.1 Clear identification of potential types of adverse effects, complications, or errors is important for decision-making processes as they may be related to different local, systemic, and technical aspects.4 Likewise, these complications will influence the predictability of treatment. As a result, knowledge of the clinical evidence regarding adverse effects, errors, and complications is required and cannot be ignored. Therefore, the present review structures the underlying biological mechanisms, clinical evidence, and clinical recommendations for the most common systemic risk factors for implant-related complications.
2.1 | Biological mechanism
Although the biomolecular mechanisms are still not well understood, it seems that the local and systemic effects of nicotine and other tobacco components may compromise bone metabolism at the bone/implant interface and jeopardize the maintenance of stable osseointegration. Several animal and in-vitro studies indicated that nicotine can inhibit the proliferation of fibroblasts and their production of fibronectin and collagen.5–9 Other studies reported that tobacco products can modulate bone turnover by increasing the production of MMP-1,2,3 in osteoblasts and, consequently, favoring the bone resorption processes.10,11 Based on current experimental evidence, an amplification of oxidative stress in periodontal and peri-implant tissues, an increase in the production of IL-6, IL-8, PGE2, and AGEs by gingival fibroblasts, and an impairment of the function of polymorphonuclear neutrophils could justify the mechanisms underlying the onset of biological complications around implants.12
2.2 | Clinical evidence
The effects of smoking constantly seem to be dose-dependent. Conversely, they might not be influenced linearly by the exposure over time.13 Indeed, two studies have shown that heavy smokers (>20 cigarettes/day) have a threefold higher risk than moderate or light smokers of experiencing implant loss, while for marginal bone loss (MBL) greater effects (especially in the maxilla) have been reported for heavy smokers compared with nonor moderate smokers.14,15 A systematic review and meta-analysis by Manzano et al.,16 in which factors that could jeopardize the establishment of an intimate bone-to-implant contact in the early stages of bone healing were evaluated, concluded that tobacco consumption is an important risk factor capable of significantly increasing the risk of early implant failure from 1.3to 2.3-fold. Furthermore, in a review by Moraschini and Barboza17 after a follow-up ranging from 8 to 240 months, a statistically significant difference in MBL in favor of the non-smoking group (standardized mean difference = 0.49, p < 0.00001) was detected. Similarly, Nazeer et al.18 found significantly higher MBL at 3, 6, and 9 months after implant loading in smoking patients compared to non-smokers. At the same time, several authors have suggested that bone loss is greater in the maxilla than in the mandible.17,19,20 When smokers were compared to non-smokers, the implantrelated odds ratio (OR) for a late failure rate of osseointegrated implants was significantly elevated, as demonstrated by Moraschini et al. (OR = 1.96), Strietzel et al. (OR = 2.25), and Hinode et al. (OR = 2.17).17,21,22 Similarly, a more recent review reported a significantly greater relative risk (RR = 2.45) for implant failure in smokers of >20 cigarettes per day compared with non-smokers.23 Finally, some authors have reported that tobacco consumption can negatively influence the survival rate of the implant after ridge augmentation procedures.22,24,25 Notably, a review by Chambrone et al.24 found a significantly higher risk of implant loss (RR = 1.87) after sinus lift in the smoking group than in the control group. A retrospective study by Kan et al.25 over a mean follow-up period of 41.6 months, found a significantly different cumulative implant success rate (p = 0.027) in grafted maxillary sinuses between non-smokers and smokers, with values, respectively, of 82.7% and 65.3%.Unlike periodontitis, the correlation between smoking and peri-implantitis seems to be controversial. A multilevel cross-sectional study by Pimentel et al.26 conducted on 147 patients observed athree times higher probability of developing peri-implantitis (prevalence ratio = 3.49) in smokers compared to non-smokers. A study by Roos-Jansåker et al.27 carried out on 218 implant patients followed up for 9–14 years concluded that smoking was a variable significantly associated with the risk of peri-implantitis in both univariate and multivariate analyses. A recent cross-sectional study by Costa et al.28 on 350 subjects reported an adjusted OR for the occurrence of periimplantitis of 2.63 for current smokers compared with non-smokers. A review by Sgolastra et al.29 revealed, with little evidence and a limited number of studies, that smoking was a significant risk factor for peri-implantitis when an implant-based meta-analysis was conducted (OR = 2.1), unlike the patient-based analysis which did not obtain the same significance (OR = 1.17). A meta-analysis by Stacchi et al.30 found no evidence justifying the role of smoking in the pathogenesis of peri-implantitis, reporting no statistically significant differences in the rate of implant loss and peri-implantitis between smokers and non-smokers. Finally, the 2017 World Workshop on the classification of periodontal and peri-implant diseases and conditions stated that the available evidence supporting tobacco consumption to be a risk factor/indicator for peri-implantitis is still inconsistent and inconclusive.31
2.3 | Clinical recommendations
Although there are no contraindications to implant placement in smoking patients, the clinician should consider the above observations. There are currently no clear guidelines indicating the daily cut-off of cigarettes that the implant patient can smoke. However, patients should be informed that the hazardous effects of tobacco maybe directly proportional to the dose and frequency of smoking habit, and therefore it should be recommended that they quit before any implant procedure. The most common reasons given to support patient obedience with perioperative smoking cessation are the potentially high costs of implant failure and understanding that implants are “an investment worth of smoking cessation”. Besides, smoking patients who are candidates for implant therapy should be advised to follow cessation programs that incorporate multidisciplinary approaches, both medical and psychological.
Some of the deleterious effects of smoking on periodontal tissues are reversible after cessation and therefore the possibility of quitting smoking can represent, a prerequisite for maintaining and preserving long-term implant health.32
3.1 | Biological mechanism
Diabetes represents a group of metabolic disorders characterized by hyperglycemia and caused by partial or total insulin deficiency or resistance. Epidemiological studies have predicted an increase in the worldwide prevalence of diabetes from 2.8% in the 2000s to 4.4% in 2030.33 The increasing prevalence of this disorder and its potential biological implications underline the importance of making some considerations before and during implant therapy for this type of patient. Several Histomorphometric and animal studies have highlighted some mechanisms that would justify an alteration of surgical wound healing and osseointegration. First, studies in pigs with streptozocin-induced diabetes reported a significant delay in wound re-epithelialization associated with a significant decrease in wound fluids of concentration of growth factors such as IGF-1 and TGF- β.34 Secondly, studies in diabetic mice have found overexpression of MMP-9 metalloproteinases in the wound microenvironment impairing granulation tissue formation and producing the inactivation of various growth factors.35 In fact, the increased amount of MMP-9 would favor a shift toward a pro-degradative activity of the fibroblasts determining the production of a lesser and texture-altered extracellular collagen matrix.36 The hyperglycemia-induced chronic inflammatory state could also have unfavorable repercussions on osseointegration. In fact, the production of advanced glycation endproducts (AGEs) and the increase in the levels of reactive oxygen species (ROS) would seem to be the basis of the mechanisms that could justify the increase in bone resorption and a decrease in its formation. First, the interaction of AGEs with their RAGE receptors (receptors for AGEs) would lead to the release of proinflammatory cytokines and the inactivation of osteoblasts. Second, systemically produced ROS would induce apoptosis of bone marrow stromal cells, osteocytes, and osteoblasts altering their differentiation processes and their ability to modulate mineralization.37 Finally, immunohistochemical studies on rats injected with AGEs found less bone-to- implant contact and a slower rate of osseointegration, which would lead to compromised implant stability.38
3.2 | Clinical evidence
Similar to smoking, findings on the implant failure and the onset of peri-implantitis rate in diabetic and non-diabetic patients appear to be controversial and not as strong as in periodontitis where diabetes is considered as a grade modifier. The biological mechanisms could explain the results of some studies that reported an increase in the failure rate and peri-implantitis in diabetic subjects. A retrospective study by Fiorellini et al. conducted on 40 (wellcontrolled) diabetic patients with 215 implants followed for an average follow-up of 8.9 ± 14.3 years, revealed a lower cumulative success rate (85.7%) than the criteria established in the literature. Notably, 24 of the 31 total implants lost in the study were found to have occurred between the first 6 and 18 months, resulting in a first-year cumulative success rate of 88.9%.39 Similarly, a review by Annibali et al., while not finding a statistically significant difference in the overall implant survival rate between diabetic and nondiabetic patients, a higher tendency for implant failure was observed in diabetic patients during the period of osseointegration and the first year after loading reporting an implant loss rate of 4% ± 1% and 3% ± 1%, respectively. After the first year, these values remained constant throughout the 6 years of follow-up. Finally, an analysis of the implant survival rate did not allow to show a statistically significant difference between diabetic (type 1 and 2) and non-diabetic patients in a review by Moraschini et al.41 (RR = 1.43, p = 0.47; RR = 3.65, p = 0.29). A difference in the implant failure rate was also not appreciated in a comparison between patients with type 1 and type 2 diabetes (RR = 1.56, p = 0.34). Also, a review by Chrcanovic et al.,42 including 14 publications, demonstrated that diabetes is unable to significantly influence the implant failure rate with a risk ratio of 1.07. Finally, by evaluating the implant failure rate over a longer time frame, a 21-year retrospective cohort study was able to conclude that a diabetic condition is significantly correlated with an increased risk of implant loss (OR = 2.75).43 A review by Al Ansari, including 89 studies with a mean follow-up of 3.2 + 2.9 years, also found a slightly higher but statistically significant relative risk of implant failure when diabetic patients were compared with healthy subjects (OR = 1.777, p < 0.001). In addition, patients with type 1 diabetes experienced failure more frequently than type 2 diabetics (OR = 4.477, p = 0.03). Conversely, some authors have not observed a significant effect of diabetes on early implant failure. An observational study by Eskow et al.45 demonstrated that diabetic patients with poor glycemic control (8.0% ≤ HbA1c ≤ 12.0%) had very high 1-year survival rates (98.6%), without the onset of any complications. Another review reported a significant association between diabetes with poor glycemic control and implant failure, with a 50% higher probability for implant failure compared to healthy patients (OR = 1.89, p < 0.001). Last but not least, the healing time is another consideration that should be kept in mind when placing implants in diabetic patients. A study by Oates et al. evaluated the effects of controlled glycemic levels on implant stability quality (ISQ) over a 1-year time. Although delayed implant integration was observed in the group with HbA1c >8.1% compared with the group with wellor moderately controlled glucose levels, no association between ISQ and HbA1c was appreci- ated in the 1-year post-loading period. Concerning the mean difference (MD) of MBL around implants, a review by Al Ansari et al.44 reported a significant difference (MD = 0.776 mm, p = 0.027) between diabetic and non-diabetic patients in favor of the latter. A meta-analysis by Chrcanovic et al.42 detected an MD (0.20 mm, p < 0.001) for MBL which, although significantly greater in the diabetic group, was almost clinically irrelevant. Also in a review by Moraschini et al.,41 in which 5 included studies reported outcomes for MBL, they found a minimal but statistically significant MD (0.18, p < 0.00001) favoring the non-diabetic group.
Regarding the incidence of peri-implantitis, a review by Dreyer et al.48 noted that patients with type 2 diabetes mellitus have more than double the risk than non-diabetic patients for developing peri-implant disease (OR = 2.5). However, most of the studies included in that review were cross-sectional compromising the quality of the evidence which was rated as medium level.48 Conversely, several studies have failed to find a correlation with diabetes. A retrospective study by Alberti et al.49 conducted on 204 patients followed up for 5.7 ± 3.82 years after loading, found an adjusted OR (0.47) for diabetes as a nonsignificant risk factor for peri-implantitis. A retrospective analysis by Renvert et al.50 performed on 240 patients also failed to demonstrate a correlation between type 2 diabetes and the development of periimplantitis. Finally, the 2017 World Workshop also stated that it is not yet possible to establish with conclusiveness and consistency whether diabetes represents an effective risk factor for the onset of peri-implantitis.31
3.3 | Clinical recommendations
Diabetes is not an absolute contraindication to implant therapy. Generally, it can be concluded that the survival rate for implants in diabetic patients maybe slightly lower than that of healthy patients, and failure is appreciable, especially in the long term.51 Though the literature regarding survival rates and the need for glycemic control is quite inconsistent due to a lack of standardization of collected data and results, it is desirable and recommended to have well-controlled glycemic control in patients undergoing implant therapy. Diabetes is defined as ‘well-controlled’ when HbA1C values are < 7%. In addition to this parameter, which evaluates the glycemic levels in the previous 2–3 months, a measurement of the pre-prandial glucose levels and the post-prandial peak could also give us a broader view of the current state.3 A good peri-operative glycemic control would guarantee better and faster osseointegration of the implants and better healing of the surgical wound. Conversely, poor metabolic control (≥8.1%) associated with diabetic microangiopathy would cause an alteration of the immune response, an impairment of the flap vas- cularization and would favor the onset of infectious complications. Based on the same rationale, prophylactic antibiotic therapy is also recommendable in diabetic patients.52 Amoxicillin is the antibiotic of choice to prevent any infections caused by streptococci, gramnegative and gram-positive anaerobes. The use of topical antiseptics (chlorhexidine digluconate 0.12%) has also proved to be useful in reducing the risk of implant failure.3
4.1 | Biological mechanism
Osteoporosis is a skeletal disease characterized by a qualitative and quantitative decrease in bone mass. According to WHO, osteoporosis is defined as a condition in which bone mineral density (BMD) is 53 The microstructural alterations of the bone tissue, such as increased cortical bone porosity and thinning of the medullary trabeculae, causes bone deterioration and consequently an increase in the risk of fractures.In 80% of cases, this condition is diagnosed in women, as post- menopausal estrogen decline causes an acceleration of bone resorp- tion. At the same time, the chronic use of some medications (such as glucocorticoids, antiepileptics, immunosuppressive drugs, proton pump inhibitors, Selective Serotonin Reuptake Inhibitors), and some systemic conditions (diabetes mellitus, severe hyperthyroidism, Cushing’s syndrome, severe hepatic disease, multiple myeloma, leu- kemia, lymphoma, hemophilia, rheumatoid arthritis) may be associ- ated with the development of osteoporosis.54
The success of implant therapy and osseointegration are strictly conditioned by the bone quality of the jaws and it is for this reason that osteoporosis could represent a condition of important clinical relevance. Type IV bone with poorly represented cortical and tra- becular bone was shown to be more frequently associated with im- plant failure; due to difficulty obtaining primary stability.55
However, the relationship between skeletal and mandibular or maxillary bone density is limited and inconsistent.56,57
Indeed, some authors have found a weak evidence to establish that regional bone density could be a useful parameter in predicting primary implant stability and at the same time a useful indicator of skeletal BMD.56,58
4.2 | Clinical evidence
In response to these issues, Merheb et al., in fact, evaluated the quality of implant stability (ISQ) at the time of implant and abutment placement using a resonance frequency analysis in osteoporotic and non-osteoporotic patients. Although a statistically significant difference in quality scores at abutment placement was found between osteoporotic patients (66.4 ± 9.5 ISQ) and healthy patients (72.2 ± 7.2 ISQ), in favor of the latter, an overall survival rate of 100% was reported.59
On the other hand, a retrospective analysis by Alsaadi et al.,60 using multivariate logistic regression, found a significant association between early implant failure and osteoporosis (OR = 2.88, p < 0.001); however, a causal relationship could not be established. Interestingly, the same research group in another study using a 61 Therefore, despite the micro and macroscopic anatomical modifications linked to osteoporosis which could undermine the primary stability of the implant, the literature, albeit very lacking, does not seem to place emphasis on this issue and does not outline osteoporotic conditions as a contraindication to implant therapy.
Regarding MBL, some studies reported a significantly greater increase in osteoporotic patients than in healthy ones.62–64Notably, de Medeiros et al.62 found a statistically significant difference in MBL around implants between patients with and without osteoporosis (0.18 mm, 95% CI 0.05–0.30, p = 63 observed a mean MBL of 1.20 ± 0.29 mm in the osteoporosis group and 0.87 ± 0.15 in the control group, with a significant difference between the two groups (p = 65 which analyzed 148 implants in 48 women over an observation period of 5 years, concluded that no statistically significant differences in MBL values were obtained between the osteoporosis group and the group of healthy women at both patient level (p = 0.54) and implant level (p = 0.31).
Regarding the incidence of peri-implantitis, a 2017 World Seminar 31 A cross-sectional study by Dvorak of 203 women with 967 implants failed to identify an association between systemic bone loss and peri-implantitis (OR = 2.1, p = 66 48 62,67,68 62 considering 15 publications involving 8859 patients and 29 798 implants, found no differences in the implant survival rate between patients with and without osteoporosis, nor at the implant level (RR 1.39, p = 0.11) nor at the patient level (RR 0.98, p = 0.94). Similarly, a meta-analysis by Lu et al. and Chen et al. could not demonstrate a significant detrimental effect of osteoporosis on implant survival (RR = 1.19, p = 0.067; RR = 1.09, , p = 0.14, respectively). 67,68
4.3 | Clinical recommendations
There are no reported contraindication for implant placement in patients with osteoporosis. However, the healing process of bone tissue might take longer and the low quality of bone could affect primary stability and consequently osseointegration.69 There are no studies that provide indications on the exact healing time in these 70 In many respects, this condition would not seem to represent a limitation per se for implant therapy, but rather the necessary therapy usually made up of bone resorption inhibitors could expose the patient to serious post-surgical complications.
5.1 | Biological mechanism
Bisphosphonates (BPs) are antiresorptive medications, consisting of inorganic pyrophosphate analogs. They are used for the prevention of osteoporosis-related fractures in patients with osteoporosis or osteopenia or in the management of cancer-related fractures in patients with bone metastases associated with multiple myeloma or solid tumors (such as lung, prostate, and breast cancers). They have also been shown to be effective in the treatment of Paget’s disease and osteogenesis imperfecta.71 BPs act by suppressing bone turnover processes through the inhibition of osteoclastic activity. They exhibit a strong binding capacity to hydroxyapatite crystals and reach higher concentrations in areas of bone tissue subject to processes of neo-apposition and resorption. Because of these properties, they can persist incorporated into bone tissue for up to 10 years, increasing bone mass and mineral density and reducing the risk of fractures.72 The route of administration can be oral, intramuscular, or intravenous (Figure 1A–C). Denosumab (DNB) is the active substance of a newer antiresorp-tive medication, which finds indication generally in the same clinical scenarios as BFs. It is able, by mimicking the effects of osteoprotegerin, to bind the receptor activator of the nuclear factor-kappa B ligand (RANKL) blocking its interaction with the RANK receptor and consequently neutralizing the function and differentiation of osteoclasts and their progenitor cells.73 In particular, DNB can interact with the precursors of osteoclasts in the marrow bone tissue, interrupting their maturation and altering the activity and survival of the already differentiated osteoclasts.74 The use of antiangiogenic agents in combination with antiresorptive drugs is known to increase the risk of MRONJ development; however, little is known regarding the incidence and prevalence of antiangiogenic-related MRONJ in antiresorptive drugs-naïve individuals. Antiangiogenic inhibitors have been increasingly used in the management of a range of malignancies including ovarian cancer, metastatic renal cell cancer, breast cancer, colorectal cancer, non-small-cell lung cancer, and glioblastoma multiforme. Antiangiogenic inhibitors can be categorized into three major groups based on their mechanism of action: anti-VEGF monoclonal antibody (e.g., bevacizumab), VEGF decoy receptors or VEGF-Trap (e.g., aflibercept), and small molecule tyrosine kinase inhibitors that block the VEGF receptors downstream signaling pathways (e.g., sunitinib, cabozantinib, and sorafenib). Additionally, the mammalian target of rapamycin inhibitors also seems to have antiangiogenic effects by inhibiting the production of VEGF and plateletderived growth factors.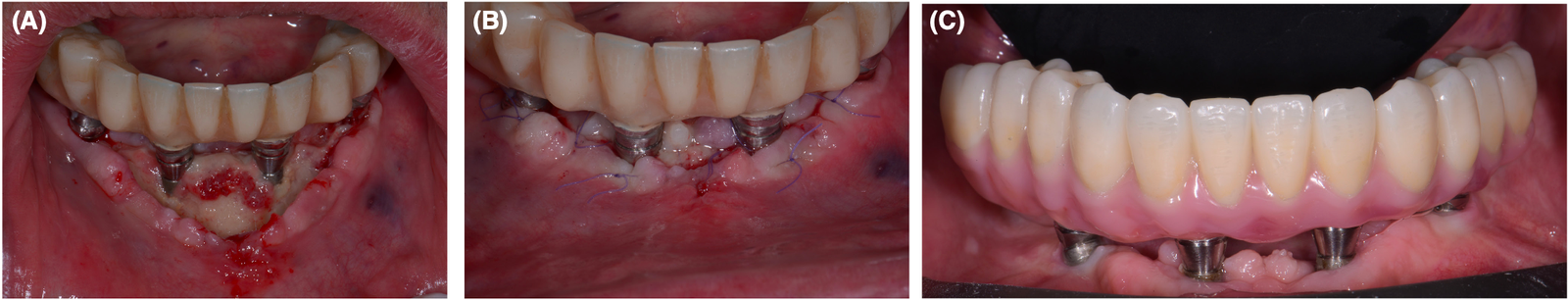 FI G U R E 1 (A) Pain, swelling and development of exposed bone in a patient taking antiresorptive medications (that were not properly investigated by the implantologist) 4 weeks after implant surgery (all on four in the lower jaw). (B) After bone debridement, the patient was treated with L-PRF membranes and prolonged antibiotic therapy (14 days of amoxicillin and metronidazole). (C) 6 months follow-up with the final prosthesis in place.
FI G U R E 1 (A) Pain, swelling and development of exposed bone in a patient taking antiresorptive medications (that were not properly investigated by the implantologist) 4 weeks after implant surgery (all on four in the lower jaw). (B) After bone debridement, the patient was treated with L-PRF membranes and prolonged antibiotic therapy (14 days of amoxicillin and metronidazole). (C) 6 months follow-up with the final prosthesis in place.
5.2 | Clinical evidence
Implant failure in patients treated with BFs and DNS needs to be investigated from several angles. Firstly, the loss of the implant could be strictly correlated with the pharmacodynamic mechanisms of antiresorptive medications which significantly reduce bone turnover, putting at risk all processes related to implant osseointegration. Second, concerns about an increased risk of implant failure in bisphosphonate users should also be seen considering MRONJ. Regarding non-MRONJ-related implant failure, a retrospective study by Memon et al.,75 in which 153 implants placed on 100 women treated with oral BFs were analyzed, a greater early implant failure (6.5%) was observed in women treated with antiresorptive drugs, even if not statistically different from the control group. Similarly, a retrospective cohort study by Yajima et al. of 25 patients with 53 total implants failed to identify a significant association between implant loss and the use of BFs. However, in the test group, 3 implants (7.7%) in 3 patients (17.6%) did not achieve osseointegration and were lost within the first year before functional loading. In contrast, a 100% survival rate was observed in the control group.76 A retrospective radiographic study by Zahid et al. reviewed the treatment records of 362 patients undergoing implant therapy. Using a generalized estimating equation analysis the authors failed to report a significant association between the use of BFs and implant failure; however, a statistically significant correlation was noted between antiresorptive therapy and implant thread exposure (OR = 3.25, p < 0.001).77 Finally, a meta-analysis by Chrcanovic et al., including 18 publications, detected an OR for late implant failure of 1.73 (p = 0.003) when patients treated with BFs were compared with patients without BFs. At the same time, antiresorptive therapy did not appear to negatively affect MBL outcomes. However, the author advises that these results should be taken with caution due to the limited number of studies included, their low specificity, and the lack of controls.78 On the other hand, with regard to the MRONJ-related complications and risk of implant loss, a recent meta-analysis reported that there is a moderate overall quality of evidence to conclude that in patients treated with BFs (either oral bisphosphonates for osteoporosis or intravenous bisphosphonates for malignancy) or DNS, the risk of developing an “implant surgery-triggered” medication-related ONJ (MRONJ) is significantly higher than in patients without antiresorptive therapy. And, furthermore, it makes sense to assert that the risk of implant failure might increase when in association with concomitant MRONJ.79 Sher et al., analyzing 830 patients (2841 implants) treated with both oral and intravenous bisphosphonates, recorded 102 cases of MRONJ with an incidence of 12.3%. However, many authors do not consider the surgical procedure of implant placement to be the trigger for MRONJ,80,81 as most of the periimplant osteonecrosis lesions are found as delayed complications on previously osteointegrated and prosthetically loaded implants.80,82 An estimate of the risk of developing ONJ In patients on antire-sorptive therapy requires a comprehensive assessment of the patient’s general and dental history. Indeed, several systemic and local risk factors could predispose to the development of MRONJ. First, the type of drug, the route of administration, and the duration of the therapy over time would seem to influence its onset. Indeed, some authors have suggested that the risk of MRONJ is significantly lower in patients treated with oral BPs than in those treated with intravenous BPs,83 with an incidence of spontaneous osteonecrosis of 0.01%–0.04% and 0.8%–12%, respectively.84 Then, some studies concluded that the duration of treatment with antiresorptive drugs has a significant influence on the onset of ONJ, as a consequence of a cumulative dose load.80 Even cancer patients, usually treated with intravenous BFs (i.e. pamidronate or zoledronic acid) would seem to be at higher risk of developing ONJ than patients with benign bone diseases.83 Secondly, incongruous prostheses, the presence of periodontitis or peri-implantitis, the concomitant intake of other drugs or local anatomical factors could influence the onset of ONJ. In particular, the presence of an inflammatory and infectious condition, such as periodontitis or peri-implantitis, in association with osteoclasticmediated bone turnover alterations, could lead to an increased risk of MRONJ. In fact, a study by Pichardo et al.85 demonstrated that dental implants already placed and osseointegrated at the start of BFs therapy have an increased risk of developing MRONJ in the presence of concomitant peri-implantitis. Corticosteroids, taken concomitantly with antiresorptive therapy, also increase the risk of ONJ. Finally, in terms of localization, although both jaws may be affected by this drug-related condition, the mandible (75%) is more frequently involved than the maxilla (25%), for reasons not yet well understood.715.3 | Clinical recommendations
It is advisable to follow the latest guidelines of AAOMS 2022 for the prevention of the Medication-Related Osteonecrosis of the jaws 71 (Table 1). Special concern should be considered for suspending RANKL inhibitors in osteoporosis patients. Several studies have demonstrated a rebound increase in bone resorption following the discontinuation of DNS, resulting in an increased risk of multilevel vertebral fractures. If DNS is to be suspended, the timing and duration of the holiday should be optimized in order to minimize this risk. The planned dentoalveolar surgery can be completed 3–4 months following the last dose of DNS when the level of osteoclast inhibition is waning. It can then be reinstituted 6–8 weeks after postsurgery. This management strategy minimizes the length of the drug holiday while maintaining a favorable environment for bone healing. However, according to a recent consensus conference any planned dentoalveolar surgeries can theoretically take place without restrictions utilizing the following drug holiday with a “delayed dosing window” that lasts about 2 months, starts ideally 5 months after the last dose of denosumab and ends at the beginning of the 7th month.87 Finally, other measures brought to light by the AAOMS in 2022 suggest the use of prophylactic antibiotic therapy,88–90 the use of rinses with antiseptics, the closure of surgical wounds by first intention, and the maintenance of good oral hygiene.71 A meta-analysis by Ata-Ali et al.91 demonstrated that antibiotic prophylaxis reduces the implant failure rate by 66.9%. In addition to controlling any infectious or inflammatory foci in the oral cavity, smoking cessation and good glycemic control in diabetic patients would also be recommended as a preventive strategy for MRONJ. Furthermore, in patients undergoing implant therapy, careful monitoring and clinical and radiographic surveillance should be ensured throughout the healing phase and in the following years. Antiangiogenic inhibitors have the potential to increase the risk of MRONJ development and should be carefully assessed and their suspension should be considered according to oncologist recommendation in order to slow down the progression of the MRONJ.92| Pretherapy (Nonmalignant disease) |
|
| Pretherapy (malignant disease) |
|
| During antiresorptive therapy (nonmalignant disease) |
|
| During antiresorptive therapy/ targeted therapies (malignant disease) |
|
6.1 | Biological mechanism
Implant therapy can significantly improve the quality of life in patients undergoing treatment for head and neck cancer. These patients usually undergo combined procedures involving ablative surgery and radiation therapy. Consequently, these treatment modalities, being able to involve the extraction of dental elements and determining residual bone defects of the jaws, could determine functional and aesthetic compromises of the maxillofacial mass.93 Furthermore, the sometimes-necessary radiotherapy could make subsequent reconstructive and prosthetic procedures even more complex. In fact, radiotherapy represents a clear risk factor for dental implants and bone augmentation procedures94 and at the same time, the xerostomia-induced in these patients could make prosthetic rehabilitations with mucosal support less functional and comfortable.95 Various radiation-induced alterations of soft and hard tissue could endanger implant therapy and should be taken into consideration by the clinician. In the affected tissues, exposure to radiation induces an acute inflammatory reaction characterized by the release of various proinflammatory cytokines (e.g. IL-1alpha and beta, TNF- alpha, IL-6, VEGF, etc…) with a consequent increase in vascular per- meability, thrombosis, and endothelial cell depletion.96 Most of the radiation dose (30%–40%) is absorbed by bone tissue due to its high calcium concentration.96 The earliest effects involve an alteration of the remodeling mechanisms, with an increase of the osteoclastic activity and a reduction of the osteoblastic activity. The result is a substantial qualitative and quantitative alteration of the bone tissue in general and of the architecture of the marrow microenvironment characterized by a thinning of the bone trabeculae and an increase in infiltration by the adipose cell population.97 There is also a depletion and inhibition in the differentiation of hematopoietic cells and skeletal stem cells. These imbalances in bone metabolism can predispose the patient to develop fractures and osteoradionecrosis of the jaws.98,99 Even soft tissues are compromised due to the high level of mu- cosal cell turnover, which amplifies the cytotoxic effect of radiation causing low resistance to them. The death of the basal cells of the oral mucosa leads to the onset of mucositis and ulcerations, with pain and possible bacterial superinfection.100 Finally, radiation therapy can determine fibrosis of the major sal- ivary glands, acinar atrophy, and fatty degeneration. As a result, sal- ivary flow is impaired and the patient might develop xerostomia.1016.2 | Clinical evidence
Impaired bone tissue remodeling and vascular alterations characterized by endarteritis and tissue hypoxia could plausibly put implant osteointegration at risk in patients undergoing radiation therapy. Regarding the early implant failure that could result, the data in the available literature are lacking and controversial. A retrospective study by Chrcanovic et al., using univariate binary logistic regression to assess implant failure up to abutment placement at the patient level, failed to identify a potential correlation between early implant loss and radiation therapy (OR = 1.07, p = 0.92). On the contrary, a review by Colella et al., while finding in the final follow-up an equal implant survival rate in irradiated and non-irradiated patients, recorded most implant losses in the first 36 months, and between 1 and the 12th month after placement. In agreement, a retrospective study by Granstrom et al., conducted on 631 irradiated cancer patients followed for an observational period of 25 years, reported a higher number of implant failures after the first surgical phase and before prosthetic loading or in the long term after irradiation. Regarding long-term implant survival, there is currently good homogeneity in the literature among the studies that have demonstrated a significantly increased risk of implant loss in patients undergoing radiation therapy.102 Although the current research reports good 5-year implant survival rates (84.3%–92.9%) in patients with irradiated sites, several reviews demonstrate a significantly higher implant failure rate than in patients without radiation therapy. Gupta94 found a strongly significant correlation between implant failure rate and irradiated sites in a follow-up between 6 and 120 months, with an OR of 2.95 (p < 0.00001). At the same time, the authors reported a comparable cumulative survival rate between the two groups at 7–10 years. Doll et al.,103 including 157 patients (830 implants) followed up to 20 years, demonstrated a 1.9-fold increased risk of implant loss in patients treated with radiochemotherapy compared to patients undergoing only ablative surgery. In a recent meta-analysis, Lu et al.68 established an obvious correlation between radiotherapy and implant failure, identifying a RR of 2.09. Ihde et al.96 concluded that, considering only studies reporting a statistically significant difference, implants placed in irradiated bone have a 2–3 times higher risk of failure than untreated sites. Several factors can then influence the efficacy and survival rates of dental implants in patients’ candidates for radiation therapy. First, the anatomical location of the implants would appear to be an important risk factor. Studies report a double risk of failure for implants placed in the maxilla rather than in the mandible.96,104 In particular, Ilhe et al.96 comparing maxillary and mandibular implants revealed an adjusted RR of 1.79 in favor of mandibular ones. Chambrone et al. and Javed et al. reported a significantly increased probability of implant loss at maxillary sites, with a 496% (Risk ratio: 5.96, p < 0.0001) and 300% higher failure risk compared to the mandible. In fact, the greater primary stability achieved following implant placement in the mandible could justify the results, if an early failure is considered.24,105 Conversely, better long-term results could be expected in the maxilla, as better secondary stability and a higher percentage of vascularized marrow could mitigate the adverse delayed vascular effects of radiation.106 Some authors (Shugaa-Addin 2016, Nooh 2013) have also identified a significant reduction for implant survival rate in irradiated grafted sites compared to irradiated native bone, due to lower vascularity and density of regenerated bone.107,108 Second, the radiation dose affecting the bone tissue appears to be another risk factor that may affect the success of implant therapy and a radiation dose–response relationship appears to exist with the rate of loss. Animal studies have demonstrated a decrease in implant survival as the average administered dose increases,109 but no univocal and precise cut-off out 38 Gy.110 Similarly Schoen et al.111 showed a higher risk with doses >40 Gy. Ihde et al.96 and Esposito et al.,112 however, associated a higher failure rate with doses >50 Gy and >55 Gy, respectively. Sammartino et al.,104 in a 36-month longitudinal and multicenter study, also obtained a survival rate of 78.6% and 93.6%, for implants placed in sites irradiated with doses >50 Gy and < 50 Gy, respectively. In contrast, Visch et al. reported a lower survival rate for implants placed in bone with a history of less than 50 Gy compared to those with a dosage >50 Gy.113 Furthermore, the amount of radiation also appears to influence the risk of Osteoradionecrosis (ORN) associated with implant therapy. Lee et al.110 established a cut-off not to be exceeded in order not to run into the ORN risk of 61.5 Gy; other authors, however, reported values of 50–55 Gy.113,114 Thirdly, the timing of implant placement from radiation therapy seems to influence the quality of implant therapy, even if the results in the literature are still quite controversial. Chrcanovic et al. found no difference in the survival rate of implants placed before or 12 months after RT. Similarly, Ihde et al. did not find any correlation between implant survival rate and fixture placement time. Other studies have suggested not to wait less than 12 months otherwise adequate osseointegration may not be achieved.104,115,116 Other studies recommend waiting a period of at least 6 months after RT, since restoration of osteogenesis and vascularization takes place after 3–6 months.117,118 Other authors have concluded to wait at least 24 months from the last irradiation, while others, on the contrary, have demonstrated a lower implant survival after 2 years due to the delayed effects of RT on the vessels of the irradiated area.119,1206.3 | Clinical recommendations
Implant therapy should only be considered a relative contraindication in patients undergoing radiotherapy. A careful overall assessment of all the factors that could influence the treatment should be carried out in advance, to reduce the related risks and avoid the onset of complications. A thorough interview and informed consent should be guaranteed to the patient, to illustrate both the benefits obtainable from an improvement in quality of life and the greater risk of implant failure and development of ONR related to irradiated bone sites compared to non-irradiated ones. In general, the implementation of implant therapy is not recommended during RT. Some considerations must be kept in mind regarding the implant placement before (primary placement) or after (secondary placement) RT.121 The use of implantology is advised and considered safer if implemented at least 14 days before RT. However, primary placement may have some disadvantages, related to possible implant failure due to cancer recurrence or the risk of backscatter which can weaken the radiation therapy and increase the concentration of the dosage in the tissues immediately surrounding the implant.122 Conversely, secondary placement could be related to all the complications, such as failure and ORN. If implant therapy is not feasible before RT, it is advisable to wait for a period of 6–18 months before implant placement. During this time, the acute effects of RT have worn off, normal healing mechanisms have resumed, and delayed side effects have yet to occur. Due to compromised bone remodeling and osseointegration processes, it is advisable to wait an additional time of 3 months compared to the normal times before proceeding with the second surgical uncovering phase.121 Another factor that the clinician must take into consideration is the dosage of exposure. Anderson et al.’s guidelines for:- < 50 Gy: indicate a low risk of implant failure and recommend the adoption of standard precautions.
- 50–65 Gy associate moderate risk and recommend caution.
- 65–74 Gy indicate a relatively high risk and advise against the placement of implants unless hyperbaric oxygen therapy is also used.
- 75–120 Gy implant therapy is not recommended due to the high associated risks.121
7.1 | General considerations
A cross-sectional analysis in the North American population reported a 2.8% prevalence of temporary or chronic immunosuppressive conditions.123
A state of immunosuppression can be associated with a wide variety of circumstances, ranging from pathological conditions to physiological states (i.e., pregnancy). Most cases of short-term or long-term non-HIV-related changes in the immune system that the oral or maxillofacial surgeon may face in the treatment of implant patients could derive from the intake of drugs.124
Steroid derivatives (i.e., corticosteroids) are medications used for a wide range of pathologies, including asthma and especially autoimmune diseases (i.e., rheumatoid arthritis, pemphigus vulgaris, lupus erythematosus, Sjogren’s syndrome, polymyalgia rheumatica). Immunomodulatory drugs are also used in patients with transplanted organs, for whom in addition to the use of corticosteroids (CS), other drugs such as cyclosporine, tacrolimus, sirolimus, and mycophenolate are also used.124
One of the side effects mainly related to the prolonged use of corticosteroids is an alteration of bone metabolism, so much so that it represents one of the three leading causes of osteoporosis. In fact, CSs induce apoptosis of most osteoblastic cells and osteocytes and inhibit the differentiation of stem cells into osteoblasts and prolong the lifespan of osteoclasts.125 CSs also cause a reduction in the production of insulin-like growth factor (IGF-I) by the osteoblastic cell population, compromising its anabolic and proliferative activity and the formation of new bone. At the same time, it causes an increased release of RANK ligand from osteoblastic cells, which in turn has a potentiating action on osteoclastic activity.126 The result is an imbalance between the osteoblastic-mediated activity of bone neoapposition and that of osteoclastic-mediated resorption, in favor of the latter, causing a reduction in the volume and density of the bone tissue.
The same effect was found among the side events of cyclo-sporine, as observed in a rat Histomorphometric study by Shen et al.127 Another animal study demonstrated that cyclosporin, thanks to its ability to induce the production of TGF-beta, can determine suppression of MMP 2 and −9 in rats. As a consequence, there is an impairment of the processes of angiogenesis and bone remodeling, and healing.128
7.2 | Clinical evidence
Although the mechanisms described above justify the intentions of some studies to investigate the plausible correlation between the administration of these drugs and implant failure, the scarce literature available to date seems to have failed to out 129 A review by Duttenhoefer et al., in which 19 studies (11 case reports, 6 retrospective, and 3 prospective) were included, investigated the influence of autoimmune diseases and their treatment with steroid derivatives on implant loss over a period ranging from 6to 156 months of follow-up. In none of the studies did the comorbidity associated with these conditions influence implant failure, reporting a survival rate of 88.8%.124 A retrospective study by Petsinis et al., in which 31 patients (105 implants) receiving glucocorticoid therapy for various systemic diseases were followed up for 3 years, observed an implant survival rate of 99% and did not find any signs clinically or radiographically relevant evidence of impaired osseointegration.130
Similarly, in the review by Duttenhoefer et al., in which 6 studies of patients with organ transplants and immunosuppressive therapy were included (2 prospective and 4 case reports), an implant survival rate of 100% was reported at a mean follow-up of 58 months and no clinically appreciable effect on osseointegration was found. A recent systematic review and meta-analysis by Burtscher et al., however including only case–control studies and case series/case reports, found a 100% implant survival rate in transplant patients receiving various drugs for immunosuppressive therapy at an average follow-up of 60 months. At the same time, no studies described early implant loss, and no statistically significant differences were reported for MBL between transplant recipients and healthy control patients.
7.3 | Clinical recommendations
Although the quality of the available evidence is very low, above all due to the prevalent presence of non-controlled and nonrandomized studies, the results we have available today seem to find no contraindication to implant therapy in patients with drug-induced immunosuppression and organ transplants.124 New investigations are certainly necessary to increase the data at our disposal and to highlight the aspects of such a vast and articulated research field.
Generally, to avoid peri-operative complications in patients on prolonged corticosteroid therapy, some considerations must be made. Indeed, steroid-derived drugs could cause some level of suppression of the hypothalamic–pituitary–adrenal axis, rendering the patient unable to produce cortisol in stressful occasions such as surgical procedures. This could trigger an adrenocortical crisis manifesting in hypotension and collapse.131 To prevent this event, an administration of a supplemental dose of corticosteroids to the patient 30 min before surgery has been suggested. However, the available evidence has shown that the risk of adrenocortical crisis is very low in patients on prolonged steroid therapy undergoing minor surgery and, therefore, supplemental steroid therapy is unnecessary.132
Finally, since immune-suppressed patients are a category to be considered at high risk for postoperative infections, prophylactic antibiotic therapy should always be contemplated. A 5–6 day protocol depending on the type of drug (first choice amoxicillin + clavulanic acid or clindamycin or moxifloxacin in case of allergy), to be started 12–24 h before implant placement, is considered adequate.133
New investigations are certainly necessary to increase the data at our disposal and to highlight the aspects of such a vast and articulated research field.
8.1 | Biological mechanism
HIV is the pathogen of an infectious condition which in its final stages can lead to AIDS. CD4+ T helper lymphocytes represent the cell population that expresses the greatest number of surface receptors for HIV. Their depletion, which typically occurs in AIDS, occurs through a direct cytolytic effect of the virus and through an immune attack on virus-infected cells.134 The virus is also capable of invading macrophages and dendritic cells, causing a progressive collapse of the immune system. The reduction in the lymphocyte count results in an increased susceptibility of the patient to develop opportunistic infections and malignancies. Furthermore, the lack of inhibition of viral replication can predispose to the development of cardiovascular, hepatic, renal, neurological, and tumoral diseases, significantly compromising the life expectancy of the HIV+ patient.135 With the introduction of highly active antiretroviral therapy (HAART) and combined antiretroviral therapy (cART), mortality from AIDS has drastically reduced, allowing stabilization of the patient’s immune status and an improvement in quality of life. Several mechanisms in the HIV+ patient may explain an increased risk of implant therapy failure. In addition to immunodeficiency which may predispose to infectious conditions in the oral cavity, chronic inflammation, immune dysregulation and antiretroviral therapy itself may impair bone tissue metabolism.136 In vitro studies have demonstrated that several HIV-associated viral proteins can promote osteoclastic activity and the release of proinflammatory cytokines (i.e. IL-6, TNF-α) which induce osteoclastogenesis and bone resorption.137,138 Furthermore, the viral ribonucleic acid (RNA) load is correlated to an increase of plasmatic concentration of RANKL capable of stimulating osteoclastic activity and altering bone turnover.139,140 In addition, several drugs that are part of HAART, including protease inhibitors (PIn), can also affect BMD resulting in osteopenia and osteoporosis.8.2 | Clinical evidence
Several studies have highlighted that the CD4+ T cell count and the implementation of antiretroviral therapy are two very important factors capable of influencing implant therapy in HIV+ patients. May et al., in a prospective study that monitored AIDS-only patients with CD4+ T cell count < 200 cells/μL for 5 years, reported a slightly higher implant failure rate (10%) for HIV+ patients compared to healthy patients (5%–7%).141 Other authors have reported a higher incidence of postoperative complications in patients with severe immunodeficiency (CD4+ T cell count < 200 cells/μL) who underwent oral surgical procedures in general.142,143 Neumeier et al.,144 at 3 years, in HIV+ patients with a controlled CD4 count (mean CD4+ T cell count: 603 cells/μL), cART therapy, antibiotic prophylaxis, well-designed prosthetic restorations, and regular 6-month maintenance found rates of implant survival and MBL compared to those of healthy patients. Similarly, Vidal et al.,145 in HIV+ patients with CD4+ T cells >350/mm3 showed an eventless implant healing, an implant survival rate of 100% and the same incidence of peri-implant disease (mucositis/peri-implantitis) of healthy patients. Sivakumar et al.,146 also reported a good implant survival rate (95%) in AIDS patients in the short term (2.8 years). A systematic review by Ata-Ali et al.,135 in which 7/9 of the included studies evaluated HIV+ patients with CD4+ T cells >350/mm3, reported good and similar prognostic values for implant therapy between AIDS patients and healthy ones. A review by Lemos et al.,147 in which 5/6 included studies evaluated patients with CD4+ T cells >400/mm3, reported survival rate values for HIV+ patients (95%) in line with those of the healthy population. As far as peri-implant diseases are concerned, Casula et al. found a peri-implantitis prevalence of 34% in HIV+ patients who underwent single crown/bridge implant rehabilitations; it was possible to correlate the variable relating to the immunological profile (CD4+ and CD8+ counts) of the patient exclusively with peri-mucositis disease, but not with peri-implantitis. Only ‘age’ has been identified as the main risk factor for implant loss.148 Other authors, however, have highlighted that the risk of periimplantitis and implant failure could be correlated with other factors not related to immunodeficiency. Gay-Escoda et al.,149 in fact, although only patients with CD4+ T cells >250/mm3 were analyzed, recorded a high prevalence of peri-implantitis (45%) diagnosed above all in non-compliant patients and with a history of advanced periodontitis. Sabbah et al, in another 5-year retrospective analysis, found similar implant failure (HR = 1.4, p = 0.34) between HIV+ and HIV− patients. However, an adjusted analysis demonstrated a significant correlation in HIV+ patients between implant loss and various risk factors, such as CD4+ T cells < 20% (HR = 2.72, p = 0.04), smoking (HR = 2.61, p = 0.05), PI administration (HR = 2.74, p = 0.04) and implant placement in the anterior maxilla (HR = 5.82, p < 0.001). The concomitant presence of these 4 risk factors determines a drop in the implant survival rate to 58.3%.150 Finally, a recent study with 12 years of follow-up, evaluated the possible side effects of cART influencing the failure of implant therapy. In fact, due to mechanisms that are not yet well understood, antiretroviral therapy can favor the onset of osteopenia, osteoporosis, and a lowering of BMD. In particular, the risk of developing osteopenia appears to be 2 times greater in patients treated with PIs than in patients who do not use them.151 However, Oliveira et al, reported mean MBL values after a 7-year follow-up of 2.43 (1.48) mm, which are consistent with physiological peri-implant bone remodeling processes and are similar to those recorded in a healthy population in a 10-year follow-up.151,1528.3 | Clinical recommendations
Although the available evidence is lacking and sometimes inconsistent in defining clear guide patients undergoing implant therapy, the use of dental implants is generally not contraindicated in patients with AIDS. Long-term maintenance of CD4+ T lymphocyte levels >250 cells/mm3 appears to be an important factor that can mitigate the risk of impaired osseointegration and implant failure rates in these patients.151,153 Furthermore, studies, albeit with controversial results, seem to establish that an ideal control of major risk factors such as smoking habits (>10 cigarettes/day), uncontrolled periodontitis, poor compliance in maintaining hygiene can guarantee the success of implant therapy even in HIV+ patients. Antiretroviral therapy is not a contraindication to oral rehabilitation treatments.150,151 There is no strong evidence demonstrating an increased susceptibility of HIV+ patients to local post-surgical infections.154 In fact, usually, the implementation of HAART guarantees a potentiation and an increase of circulating hematopoietic T lymphocyte count and polymorphonuclear granulocyte count are < 200 cells/μL and < 500 cells/μL, respectively. According to the guide + patients who use intravenous drugs, due to their greater susceptibility to developing bacterial endocarditis. The use of mouth rinses with antiseptics may also be recommended in these cases.154 Because of the immune thrombocytopenia that can affect patients with AIDS, an evaluation of hemostatic function would be advised in patients who will undergo extensive surgical procedures. A platelet count < 60 000/mm3 or hemoglobin levels < 7 g/dL should lead to surgery postponement.154,1559.1 | General mechanisms
The term cardiovascular disease (CVD) indicates a broad spectrum of diseases affecting the heart and blood vessels of the entire body and represents the main cause of death in the USA, Europe, and Asia. Among the main CVDs, we can include ischemic heart disease, angina pectoris, heart failure, aortic aneurysm, and cerebrovascular diseases, including ischemic and hemorrhagic stroke. CVDs include sex, age, and family history among non-modifiable risk factors. Among the modifiable risk factors, we mainly include hypertension, atherosclerosis, hypercholesterolemia/dyslipidemia, diabetes, obesity/sedentary lifestyle, and smoking habits. In most of its forms, CVDs occur following an obstruction, a reduction of the lumen, or a rupture of medium and large arterial vvessels consequently compromising the perfusion of downstream tissues which go against necrosis from hypoxia and lack of nutrients.156 Hypoxia manifests itself with an alteration of the homeostatic mechanisms of tissue oxygenation which results in an impairment of the healing processes, with a reduction in fibroblast proliferation, collagen synthesis, capillary growth, and macrophage activity.157 Due to the nature of these mechanisms, it has been hypothesized that CVD may alter bone healing mechanisms around implants. Therefore, it was considered plausible to investigate a possible correlation between CVD and impaired osseointegration.
9.2 | Clinical evidence
Several retrospective studies with follow-up from 7.3 up to 21 years, did not find a significant increase in the implant failure rate in cardiovascular patients.43,60,157–159 No influence (p = 0.359) of coexisting CVD was appreciated on the implant survival rate of diabetic patients in a retrospective study by Araujo Nobre.160
Surprisingly, a review by Wu et al. reported the positive effect of antihypertensive drugs on implant survival. An analysis of survival adjusted for confounders obtained favorable results in favor of users of antihypertensive drugs (HR = 0.12, p = 0.01). Beta-blockers, thiazine diuretics, and ACE inhibitors, in fact, would seem to positively influence bone metabolism, stimulating its formation and reducing its reabsorption.161 Conversely, a review by Schimmel et al. and several retrospective studies unanimously demonstrated that there is no evidence regarding hypertension as a risk factor for implant failure.162–165
Regarding the incidence of peri-implantitis, a retrospective study by Renvert et al.50 found a history of CVD in 27.3% of patients with peri-implantitis and only 3% of patients in the healthy implant/ mucositis group reporting an OR (adjusted for age, gender, and smoking) of 8.7. Saabi et al.158 through a retrospective assessment found the presence of CVD in 26% of patients with peri-implantitis. Conversely, Neves et al. failed to establish a significant correlation between cardiac disease and peri-implantitis (OR = 1.14, p = 0.61). Froum et al.,166 in a systematic review of the literature, concluded that inconsistency of data in the literature and the heterogeneity of the evaluated populations make further investigations necessary to establish the existence of a link between CVD and peri-implant disease. Similarly, there is currently no evidence demonstrating that CVD was or is not an actual risk factor for implant loss.
9.3 | Clinical recommendations
However, the clinician should take into consideration a few other issues before undertaking any surgical treatment in this category of patients. In fact, an increased risk of bleeding, a rise in blood pressure, and the appearance of ischemic attacks during surgery could occur. Comprehensive informed consent, in which the aforementioned risks are explained, continuous monitoring during surgery, and periodic updating of the medications taken by these patients are highly recommended.167
First, there is an absolute contraindication to proceeding with implant placement in patients with recent myocardial infarction or cardiovascular attack up to 6 months after preliminary care; in fact, harmful complications can probably occur in this time window. Similarly, patients undergoing heart valve replacement surgery should not undertake implant therapy before 6 months.3 In any case, adequate antibiotic prophylaxis must be performed to prevent the development of endocarditis.
Another aspect to carefully evaluate is the possible intake of anticoagulants or antiplatelet drugs. When we are going to treat patients treated with vitamin K or direct oral anticoagulants (DAOs), an assessment of the INR is always recommended. INR values necessarily established between 2 and 4 do not contraindicate implant placement associated with minor surgical procedures.168 Obviously, a weighing of the type of surgery that is planned is always necessary, considering that less invasive procedures are associated with a lower risk of bleeding while more complex interventions such as the insertion of bimaxillary or zygomatic implants and the use of bone grafts correlate at a higher risk.
In patients on antiplatelet therapy (AT) (i.e., acetylsalicylic acid, clopidogrel, ticlopidine, prasugrel, and ticagrelor) an adequate evaluation of hemorrhagic risk should be done by assessment of platelet count and hematocrit. Platelet counts < 50 000 cells/mm3 are associated with a higher risk of postoperative bleeding, while values < 20 000 cells/mm3 also predispose to spontaneous mucosal bleeding. On the other hand, hematocrit levels < 60% of normal values should be carefully evaluated.169
Several systematic reviews do not recommend discontinuing DAO or AT therapy before undertaking oral surgical procedures. Kämmerer et al.170 concluded that in patients with INR < 4 treated with vitamin K antagonists dental surgery procedures can be performed safely without suspending therapy, implementing local hemostatic measures. Similarly recent reviews found a clinically insignificant risk of periand post-operative bleeding in patients who have not stopped anticoagulant therapy.171,172 Analogous results have been achieved in studies of patients on AT.173 Iwabuchi et al., in a cross-sectional multicenter observational study, carried out on 2817 patients, reported a low absolute incidence of bleeding events in the anticoagulated group, but slightly higher than in untreated patients. In particular, age < 65 years, high INR values, and the presence of acute inflammation before extraction surgery could significantly increase the risk of bleeding.174
Furthermore, some authors also report a greater risk of thromboembolic events or myocardial infarction which may derive from the interruption of therapy with DAO or with antiplatelets, respectively.175,176 At the same time, other studies showed that the implementation of bridging therapies (i.e. heparin) can increase bleeding events compared to patients who continued the canonical anticoagulant therapy; no differences, however, have been.177,178
Finally, among the local measures to ensure hemostasis in these patients, the use of compressive sutures, the application of hemostatic dressings (collagen, absorbable sponges, oxidized cellulose, bone wax, and L-PRF) or topical tranexamic acid (5%, three to four times/day) can reduce periand post-operative bleeding complications and make the procedure safer.104,179
10.1 | Biological mechanisms
Chronic alcoholism is defined as voluntary excessive ingestion of ethyl alcohol. It is a primary chronic disease related to genetic, environmental, and lifestyle factors, implying serious consequences from both a health and psychosocial point of view.180 A national survey on drug use and health estimated the alcoholism rate in the population of North America at 10%.181 The parameter that allows quantifying the alcohol content in a certain volume of alcoholic beverage is the so-called alcoholic unit (UA). One unit corresponds to 12 g of ethanol, which is equivalent to the amount contained in a medium-alcoholic glass of wine (125 mL), a medium-sized can of beer (330 mL), or a shot of liqueur (40 mL).182 Various investigations have highlighted the negative effects of alcohol on various human tissues and organs, including the liver, marrow, brain, heart, and musculoskeletal system In particular, animal studies observed a significant reduction in trabecular bone volume, bone mineralization, and bone matrix synthesis in rats chronically exposed to alcohol.183,184 Friday & Howard, evaluating the effects of alcohol on cultured normal human osteoblastic cells, reported a dose-dependent reduction in protein and DNA synthesis. A reduction in the mitogenic effect of sodium fluoride and insulin-like growth factor type II was also found in these cell cultures185; moreover, some substances contained in alcoholic beverages, such as nitrosamines and ethanol, can promote bone resorption and inhibit the stimulation of bone formation.186 A rabbit study by Koo et al.187 found that an alcoholic diet caused a significant reduction in bone formation, bone density, and bone-to-implant contact. Biochemical and Histomorphometric evaluations revealed a significant impairment caused by habitual alcohol exposure on primarily osteoblastic cell function and proliferation, while osteoclastic activity would be unaffected.188 Thus, alcohol-induced bone disease results in a reduction of apposition processes in favor of resorption processes. The decoupling of these two physiological mechanisms results in defective remodeling and reduction of skeletal tissue mass, increasing the risk of fractures. These mechanisms justify the link between habitual alcohol consumption and osteopenia. In vivo and in vitro studies also showed an alcohol-induced weakening of the immune system. In particular, it has been observed that ethanol can inhibit the key functions of polymorphonuclear neutrophils, determining through a timeand dose-dependent mechanism a reduction of IL-8 production and mRNA synthesis.189 Finally, excessive chronic alcohol consumption can indirectly lead to coagulation defects due to secondary liver cirrhosis. In addition to a reduction in the production of all factors necessary for hemostasis, cirrhosis can also lead to impaired platelet distribution and result in hypersplenism and folate deficiency.19010.2 | Clinical evidence
Several clinical studies, therefore, have deemed it appropriate to investigate the possible correlation between alcohol consumption and peri-implant bone resorption. In a prospective clinical study by Galindo-Moreno et al., through a multivariate linear regression anal- ysis, alcohol use, implant surface area, and gingival index were iden- tified as variables significantly associated with peri-implant bone loss. In addition, the mean MBL adjusted for implant surface and the gingival index was significantly greater in alcohol users (1.49 mm) than in non-users (1.23 mm).91 Block et al. in a retrospective case–control study used backward variable selection to predict whether an implant failed within 1 year, 1–4 years, or after 4 years in 3 multivariable logistic regressions. In addition to other factors, alcohol was also associated with an increased likelihood of implant loss within 1 or more periods.191 A matched case–control analysis by Alissa & Oliver found a higher incidence of implant failure in patients who consumed >5 units/day of alcohol compared with non-drinkers or consumers of less than 5 units/day.19210.3 | Clinical recommendations
Currently, the available evidence is not sufficient to state that alcoholism is a contraindication to implant therapy. However, patients who undergo this therapy could face a series of complications relating to altered bone metabolism, bleeding problems deriving from liver pathologies, and an increased susceptibility to infection due to compromised immune systems.11.1 | Biological mechanism
Many oral problems are related to an imbalance of antioxidants and ROS in the body. Recently, free radicals are related to the occurrence and development of dental diseases, and antioxidants have also been used in dental treatment.193
Oxidative stress is an imbalance in the production of free radicals and in the antioxidant system (2), so it could be measured by monitoring some useful parameters: ROS, Malondialdehyde (MDA), Superoxide Dismutase and Total Antioxidant Capacity (TAC).193–195 All these parameters are present also in peri-implant crevicular fluid (PICF).195
On one side, ROS are required for cell signaling and normal metabolism. On the other hand, excessive oxidative stress may lead to damage to DNA, RNA, and proteins.196 Short-term oxidative stress may occur in tissue loss such as trauma, heat damage, and infection. In these damaged tissues, the production of enzymes (rich of free radicals) is increased by phagocytic cells with the release of free metal ions, epoxidation, and production of excessive ROS.197,198
When ROS production increases or antioxidant capacity decreases there’s oxidative damage to cellular components as in proteins, lipids, and nucleic acids.199
It has already been proven that oxidative stress is related to many diseases, including CVD, diabetes, rheumatoid arthritis, cancer, and various kinds of inflammation.200
Oxidative stress and oxidative damage are in the contest of dental procedures and it has been shown that periodontal inflammation is a direct result of increased ROS and oxidative damage products in the oral cavity.201
Alcohol consumption and nicotine exposure can increase ROS and are related to periodontal disease. A lot of dental procedures such as implants, bleaching, and fillings are related to oxidative stress.202–204
11.2 | Clinical evidence
The process of dental implant implantation inevitably generates ROS. Tsarik et al.205 reported that the oxide layer generated on the surface of titanium alloy implant may reduce the potential corrosion. Friction during implantation would lead to the metal surface rupture and the titanium dioxide layer corrosion and an electrochemical reaction with free radicals and hydrogen peroxide were generated as intermediate products. When titanium dioxide is corroded, hydrogen peroxide generated by electrochemical reaction will continue to react with titanium dioxide to form hydroxyl radicals.206 Bressan et al. analyzed the effects of titanium (Ti) particles on mesenchymal stem cells (MSCs) and fibroblasts (FU); they evaluate cell proliferation and generation of ROS and it was found that titanium particles reduced the survival time of the cells and increased the generation of ROS. Concurred by oxidative stress, bone regeneration imbalance was induced.207
The implant placement process may lead Ti particles to enter the tissue and cause inflammation. Therefore, tetracycline, doxycycline, chlorhexidine, hydrogen peroxide, and citric acid can be used to remove excess Ti particles, among which hydrogen peroxide, citric acid, and chlorhexidine are more effective.208
Also, Abdulhameed et al.209 reported that titanium dioxide nanoparticles (TiO2NPs) can induce oxidative stress, reduce osteogenesis, and damage the antioxidant defense system.
After a dental implant placement, friction and twisting could damage the oxide layer on the surface, leading to an increase of ROS and an inflammatory, such as peri-implantitis (PI).210,211
Antioxidants are choices for treatment. PI and periodontal disease are manifested by soft tissue and bone damage, in which ROS plays an important role in cell transmission, maintenance, and proliferation.212
A certain amount of ROS is already present before the dental implant is implanted 213 and the somministration of antioxidants have a protective effect on periodontal tissue and can neutralize ROS to prevent tissue damage.214 A lot of antioxidants can be obtained from diets and supplements, and supplements can be taken in addition to a diet rich in berries and vegetables.193 Some useful antioxidants are tannic acid macromolecules consisting of a central glucose molecule linked to 10 surrounding gallic acid units.215–218 Green tea and quercetin have both anti-inflammatory and antioxidant properties.219–222 Polyphenols are natural compounds with antioxidant and antimicrobial properties.223,224 The antioxidant activity of polyphenols cleans free radicals by supplying hydrogen atoms from hydroxyl groups in the phenolic ring,224 and chelating iron and other metal ions, thereby preventing the catalytic oxidation of hydrogen peroxide and superoxide to hydroxyl radicals.225
Curcumin, a polyphenolic compound can also lead to an in-crease in the level of the antioxidant enzyme glutathione peroxidase, which reduces the ROS level in cells.222 Vitamin E is a common antioxidant with the highest concentration in human mitochondria. The main action of vitamin E is to interact with superoxide in mitochondria, limiting its formation, stabilizing the mitochondrial membrane, and removing antioxidants that have been generated.226 A study indicated that adding low concentrations of vitamin E (less than 0.1%) did not affect the physical and mechanical properties and can prevent oxidation for up to 24 months post-implantation.227
At present, antioxidants in the treatment of local inflammatory reactions, such as periodontitis, and PI usually quickly disappear with ROS and other free radicals. Some studies reported the effects of new materials in which hydrogels can reduce the presence of ROS, and inhibit hydrogen peroxide and lipid peroxidation.195,228
11.3 | Clinical recommendations
There are no reported contraindication for implant placement in patients with elevated oxidative stress. However, there’s evidence that lowering the level of ROS could help the healing process of periimplant bone tissue.195,229
Studies that evaluate peri-implantitis found significant correlations between probing pocket depth, level of MDA and TAC.195
Moreover, the level of oxidative stress markers (MDA, SOD, and TAC) in PICF does not significantly change in peri-implantitis compared to healthy implants.195,212
Therefore, the prevention of possible peri-implantitis could be suppoterted by antioxidant supplementation and delivery by new gel encapsulation that is worth of studying.209,229
While the clinical relevance of Oxidative Stress is growing, we need to focus attention to how it affects the possible outcome of patient with dental implants.230 Like inflammation, it is likely to play a major role in both healing and harm. As diagnostic biomarker techniques become more, reliable and sophisticated, we need to interlace this information into new protocols of peri-operative evaluation in dentistry. The pre-operative and postoperative evaluation of oxidative stress is used in other surgical fields like colon-rectal oncology, a desirable outcome would be the application in dental implant surgery.231–234
Systemic conditions and/or an unhealthy lifestyle may directly lead to surgical complications, compromise implant/bone healing, or influence the long-term peri-implant health and its response to biologic nuisances. Similar risks may pertain to the medications taken by implant patients rather than the disease itself.
The present review shows that there are very few absolute contraindications and a full evaluation of each individual patient is of the utmost importance to avoid implant-related complications in medically compromised patients with or without unhealthy lifestyle/elevated oxidative stress.
Vissink A, Spijkervet F, Raghoebar G. The medically compromised patient: are dental implants a feasible option? Oral Dis. 2018;24:253-260.
Chappuis V, Avila-Ortiz G, Araújo MG, Monje A. Medication- related dental implant failure: systematic review and meta-analysis. Clin Oral Impl Res. 2018;29:55-68.
Hwang D, Wang HL. Medical contraindications to implant therapy: part II: relative contraindications. Implant Dent. 2007;16:13-23.
Zucchelli G, Wang HL, Chambrone L. Complications and treatment errors in periodontal and implant therapy. Periodontol 2000. 2022. doi: 10.1111/prd.12442 [Epub ahead of print].
Tipton DA, Dabbous MK. Effects of nicotine on proliferation and extracellular matrix production of human gingival fibroblasts in vitro. J Periodontol. 1995;66:1056-1064.
Giannopoulou C, Geinoz A, Cimasoni G. Effects of nicotine on periodontal ligament fibroblasts in vitro. J Clin Periodontol. 1999;26:49-55.
Jensen K, Nizamutdinov D, Guerrier M, Afroze S, Dostal D, Glaser S. General mechanisms of nicotine-induced fibrogenesis. FASEB J. 2012;26:4778-4787.
Milanezi de Almeida J, Bosco AF, Bonfante S, Theodoro LH, Nagata MJH, Garcia VG. Nicotine-induced damage affects gingival fibroblasts in the gingival tissue of rats. J Periodontol. 2011;82:1206-1211.
Lahmouzi J, Simain-Sato F, Defresne MP, et al. Effect of nicotine on rat gingival fibroblasts in vitro. Connect Tissue Res. 2000;41:69-80.
Jenkins K, Javadi M, Borghaei RC. Interleukin-4 suppresses IL-17– induced expression of matrix metalloproteinase-3 in human gingival fibroblasts. J Periodontol. 2004;75:283-291.
Bulmanski Z, Brady M, Stoute D, Lallier TE. Cigarette smoke extract induces select matrix metalloproteinases and integrin expression in periodontal ligament fibroblasts. J Periodontol. 2012;83:787-796.
Javed F, Rahman I, Romanos GE. Tobacco-product usage as a risk factor for dental implants. Periodontol 2000. 2019;81:48-56.
Bain CA. Smoking and implant failure – benefits of a smoking cessation protocol. Int J Oral Maxillofac Implants. 1996;11:756-759.
Nitzan D, Mamlider A, Levin L, Schwartz-Arad D. Impact of smoking on marginal bone loss. Int J Oral Maxillofac Implants. 2005;20:605-609.
Sánchez-Pérez A, Moya-Villaescusa MJ, Caffesse RG. Tobacco as a risk factor for survival of dental implants. J Periodontol. 2007;78:351-359.
Manzano G, Montero J, Martín-Vallejo J, Del Fabbro M, Bravo M, Testori T. Risk factors in early implant failure: a meta-analysis. Implant Dent. 2016;25:272-280.
Moraschini V, Barboza E dS P. Success of dental implants in smokers and non-smokers: a systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2016;45:205-215.
Nazeer J, Singh R, Suri P, et al. Evaluation of marginal bone loss around dental implants in cigarette smokers and nonsmokers. A comparative study. J Family Med Prim Care. 2020;9:729-734.
Haas R, Haimböck W, Mailath G, Watzek G. The relationship of smoking on peri-implant tissue: a retrospective study. J Prosthet Dent. 1996;76:592-596.
Lambert PM, Morris HF, Ochi S. The influence of smoking on 3-year clinical success of osseointegrated dental implants. Ann Periodontol. 2000;5:79-89.
Hinode D, Shin-ichi T, Yokoyama M, Fujisawa K, Yamauchi E, Miyamoto Y. Influence of smoking on osseointegrated implant failure: a meta-analysis. Clin Oral Implants Res. 2006;17:473-478.
Strietzel FP, Reichart PA, Kale A, Kulkarni M, Wegner B, Küchler I. Smoking interferes with the prognosis of dental implant treatment: a systematic review and meta-analysis. J Clin Periodontol. 2007;34:523-544.
Naseri R, Yaghini J, Feizi A. Levels of smoking and dental implants failure: a systematic review and meta-analysis. J Clin Periodontol. 2020;47:518-528.
Chambrone L, Preshaw PM, Ferreira JD, Rodrigues JA, Cassoni A, Shibli JA. Effects of tobacco smoking on the survival rate of dental implants placed in areas of maxillary sinus floor augmentation: a systematic review. Clin Oral Impl Res. 2014;25:408-416
Kan JYK, Rungcharassaeng K, Lozada JL, Goodacre CJ. Effects of smoking on implant success in grafted maxillary sinuses. J Prosthet Dent. 1999;82:307-311.
Pimentel SP, Shiota R, Cirano FR, et al. Occurrence of peri-implant diseases and risk indicators at the patient and implant levels: a multilevel cross-sectional study. J Periodontol. 2018;89:1091-1100.
Roos-Jansaker AM, Renvert H, Lindahl C, Renvert S. Nine- to fourteen-year follow-up of implant treatment. Part III: factors associated with peri-implant lesions. J Clin Periodontol. 2006;33:296-301.
Costa FO, Lages EJP, Cortelli SC, et al. Association between cumulative smoking exposure, span since smoking cessation, and peri-implantitis: a cross-sectional study. Clin Oral Invest. 2022;26:4835-4846.
Sgolastra F, Petrucci A, Severino M, Gatto R, Monaco A. Smoking and the risk of peri-implantitis. A systematic review and meta-analysis. Clin Oral Impl Res. 2015;26:e62-e67.
Stacchi C, Berton F, Perinetti G, et al. Risk factors for peri-implantitis: effect of history of periodontal disease and smoking habits. A systematic review and meta-analysis. J Oral Maxillofac Res. 2016;7:e3.
Schwarz F, Derks J, Monje A, Wang HL. Peri-implantitis. J Clin Periodontol. 2018;45:S246-S266.
Rosa EF, Corraini P, Inoue G, et al. Effect of smoking cessation on non-surgical periodontal therapy: results after 24 months. J Clin Periodontol. 2014;41:1145-1153.
Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes. Diabetes Care. 2004;27:1047-1053.
Velander P, Theopold C, Hirsch T, et al. Impaired wound healing in an acute diabetic pig model and the effects of local hyperglycemia. Wound Repair Regen. 2008;16:288-293.
Castleberry SA, Almquist BD, Li W, et al. Self-assembled wound dressings silence MMP-9 and improve diabetic wound healing in vivo. Adv Mater. 2016;28:1809-1817.
Wan R, Weissman JP, Grundman K, Lang L, Grybowski DJ, Galiano RD. Diabetic wound healing: the impact of diabetes on myofibroblast activity and its potential therapeutic treatments. Wound Rep Reg. 2021;29:573-581.
Saito N, Mikami R, Mizutani K, et al. Impaired dental implant osseointegration in rat with streptozotocin-induced diabetes. J Periodontal Res. 2022;57:412-424.
Quintero DG, Winger JN, Khashaba R, Borke JL. Advanced glycation endproducts and rat dental implant osseointegration. J Oral Implantol. 2010;36:97-103.
Fiorellini JP, Chen PK, Nevins M, Nevins ML. A retrospective study of dental implants in diabetic patients. Int J Periodontics Restorative Dent. 2000;20:366-373.
Annibali S, Pranno N, Cristalli MP, La Monaca G, Polimeni A. Survival analysis of implant in patients with diabetes mellitus: a systematic review. Implant Dent. 2016;25:663-674.
Moraschini V, Barboza ESP, Peixoto GA. The impact of diabetes on dental implant failure: a systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2016;45:1237-1245.
Chrcanovic BR, Albrektsson T, Wennerberg A. Diabetes and oral implant failure: a systematic review. J Dent Res. 2014;93:859-867.
Moy PK, Medina D, Shetty V, Aghaloo TL. Dental implant failure rates and associated risk factors. Int J Oral Maxillofac Implants. 2005;20:569-577.
Al Ansari Y, Shahwan H, Chrcanovic BR. Diabetes mellitus and dental implants: a systematic review and meta-analysis. Materials. 2022;15:3227.
Eskow CC, Oates TW. Dental implant survival and complication rate over 2 years for individuals with poorly controlled type 2 diabetes mellitus: diabetes and implant complications. Clin Implant Dent Relat Res. 2017;19:423-431.
Monje A, Catena A, Borgnakke WS. Association between diabetes mellitus/hyperglycaemia and peri-implant diseases: systematic review and meta-analysis. J Clin Periodontol. 2017;44:636-648.
Oates TW, Galloway P, Alexander P, et al. The effects of elevated hemoglobin A1c in patients with type 2 diabetes mellitus on dental implants. J Am Dent Assoc. 2014;145:1218-1226.
Dreyer H, Grischke J, Tiede C, et al. Epidemiology and risk factors of peri-implantitis: a systematic review. J Periodontal Res. 2018;53:657-681.
Alberti A, Morandi P, Zotti B, et al. Influence of diabetes on implant failure and peri-implant diseases: a retrospective study. Dent J. 2020;8:70.
Renvert S, Aghazadeh A, Hallström H, Persson GR. Factors related to peri-implantitis – a retrospective study. Clin Oral Impl Res. 2014;25:522-529.
Naujokat H, Kunzendorf B, Wiltfang J. Dental implants and diabetes mellitus – a systematic review. Int J Implant Dent. 2016;2:5.
Sykara M, Maniatakos P, Tentolouris A, Karoussis IK, Tentolouris N. The necessity of administrating antibiotic prophylaxis to patients with diabetes mellitus prior to oral surgical procedures – a systematic review. Diabetes Metab Syndr. 2022;16:102621.
Salari N, Ghasemi H, Mohammadi L, et al. The global prevalence of osteoporosis in the world: a comprehensive systematic review and meta-analysis. J Orthop Surg Res. 2021;16:609.
Otomo-Corgel J. Osteoporosis and osteopenia: implications for periodontal and implant therapy: osteoporosis and osteopenia: implications for periodontal and implant therapy. Periodontol 2000. 2012;59:111-139.
Goiato MC, dos Santos DM Jr, Santiago JF, Moreno A, Pellizzer EP. Longevity of dental implants in type IV bone: a systematic review. Int J Oral Maxillofac Surg. 2014;43:1108-1116.
Cakur B, Dağistan S, Sümbüllü MA. No correlation between mandibular and non-mandibular measurements in osteoporotic men. Acta Radiol. 2010;51:789-792.
Taguchi A. Triage screening for osteoporosis in dental clinics using panoramic radiographs. Oral Dis. 2010;16:316-327.
Pisulkar SG, Mistry RA, Nimonkar S, Dahihandekar C, Pisulkar G, Belkhode V. The correlation of mineral density of jaws with skeletal bone and its effect on implant stability in osteoporotic patients: a review of patient-based studies. Cureus. 2022;14:e27481.
Merheb J, Temmerman A, Rasmusson L, Kübler A, Thor A, Quirynen M. Influence of skeletal and local bone density on dental implant stability in patients with osteoporosis: implant stability and osteoporosis. Clin Implant Dent Relat Res. 2016;18:253-260.
Alsaadi G, Quirynen M, Komárek A, van Steenberghe D. Impact of local and systemic factors on the incidence of oral implant failures, up to abutment connection. J Clin Periodontol. 2007;34: 610-617.
Alsaadi G, Quirynen M, Michiles K, Teughels W, Komárek A, van Steenberghe D. Impact of local and systemic factors on the incidence of failures up to abutment connection with modified surface oral implants. J Clin Periodontol. 2007;34:610-617.
de Medeiros FCFL, Kudo GAH, Leme BG, et al. Dental implants in patients with osteoporosis: a systematic review with meta- analysis. Int J Oral Maxillofac Surg. 2018;47:480-491.
Tabrizi R, Mousavi F, Ghasemi S, Ozkan BT. Does osteoporosis increase marginal bone loss around dental implants in the posterior of the maxilla? Int J Oral Maxillofac Surg. 2021;50:964-968.
Wagner F, Schuder K, Hof M, Heuberer S, Seemann R, Dvorak G. Does osteoporosis influence the marginal peri-implant bone level in female patients? A cross-sectional study in a matched collective: Wagner et al. Clin Implant Dent Relat Res. 2017;19:616-623.
Temmerman A, Rasmusson L, Kübler A, Thor A, Merheb J, Quirynen M. A prospective, controlled, multicenter study to evaluate the clinical outcome of implant treatment in women with osteoporosis/osteopenia: 5-year results. J Dent Res. 2019;98:84-90.
Dvorak G, Arnhart C, Heuberer S, Huber CD, Watzek G, Gruber R. Peri-implantitis and late implant failures in postmenopausal women: a cross-sectional study: peri-implantitis in postmenopausal women. J Clin Periodontol. 2011;38:950-955.
Chen H, Liu N, Xu X, Qu X, Lu E. Smoking, radiotherapy, diabetes and osteoporosis as risk factors for dental implant failure: a meta- analysis. PLoS One. 2013;8:e71955.
Lu B, Zhang X, Liu B. A systematic review and meta-analysis on influencing factors of failure of oral implant restoration treatment. Ann Palliat Med. 2021;10:12664-12677.
Gibreel S, Gassim Mohamed H, Raj Suraj A, Anil S. Osseointegration of dental implants and osteoporosis. In: Gabrić D, Vuletić M, eds. Current Concepts in Dental Implantology – From Science to Clinical Research. IntechOpen; 2022.
Tsolaki IN, Madianos PN, Vrotsos JA. Outcomes of dental implants in osteoporotic patients. A literature review. J Prosthodont. 2009;18:309-323.
Ruggiero SL, Dodson TB, Aghaloo T, Carlson ER, Ward BB, Kademani D. American Association of Oral and Maxillofacial Surgeons’ position paper on medication-related osteonecrosis of the jaws—2022 update. J Oral Maxillofac Surg. 2022;80:920-943.
Diez-Perez A. Bisphosphonates. Bisphosphonates Maturitas. 2002;43:19-26.
Boquete-Castro A, Gómez-Moreno G, Calvo-Guirado JL, Aguilar- Salvatierra A, Delgado-Ruiz RA. Denosumab and osteonecrosis of the jaw. A systematic analysis of events reported in clinical trials. Clin Oral Impl Res. 2016;27:367-375.
Ottesen C, Schiodt M, Gotfredsen K. Efficacy of a high-dose antiresorptive drug holiday to reduce the risk of medication-related osteonecrosis of the jaw (MRONJ): a systematic review. Heliyon. 2020;6:e03795.
Memon S, Weltman RL, Katancik JA. Oral bisphosphonates: early endosseous dental implant success and crestal bone changes. A retrospective study. Int J Oral Maxillofac Implants. 2012;27:1216-1222.
Yajima N, Munakata M, Fuchigami K, Sanda M, Kasugai S. Influence of bisphosphonates on implant failure rates and characteristics of postmenopausal woman mandibular jawbone. J Oral Implantol. 2017;43:345-349.
Zahid TM, Wang BY, Cohen RE. Influence of bisphosphonates on alveolar bone loss around osseointegrated implants. J Oral Implantol. 2011;37:335-346.
Chrcanovic BR, Albrektsson T, Wennerberg A. Bisphosphonates and dental implants: a meta-analysis. Quintessence Int. 2016;47:329-342.
Sher J, Kirkham-Ali K, Luo JD, Miller C, Sharma D. Dental implant placement in patients with a history of medications related to osteonecrosis of the jaws: a systematic review. J Oral Implantol. 2021;47:249-268.
Holzinger D, Seemann R, Matoni N, Ewers R, Millesi W, Wutzl A. Effect of dental implants on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2014;72:1937.e1-1937.e8.
Walter C, Al-Nawas B, Wolff T, Schiegnitz E, Grötz KA. Dental implants in patients treated with antiresorptive medication – a systematic literature review. Int J Implant Dent. 2016;2:9.
Lazarovici TS, Yahalom R, Taicher S, Schwartz-Arad D, Peleg O, Yarom N. Bisphosphonate-related osteonecrosis of the jaw associated with dental implants. J Oral Maxillofac Surg. 2010;68: 790-796.
Yao S, Ding X, Rong G, Zhou J, Zhang B. Association between malignant diseases and medication-related osteonecrosis of the jaw (MRONJ): a systematic review and meta-analysis. J Craniofac Surg. 2023;34:669-673.
Vescovi P, Nammour S. Bisphosphonate-related osteonecrosis of the jaw (BRONJ) therapy. A critical review. Minerva Stomatol. 2010;59:181-203, 204-13.
Pichardo SEC, van der Hee JG, Fiocco M, Appelman-Dijkstra NM, van Merkesteyn JPR. Dental implants as risk factors for patients with medication-related osteonecrosis of the jaws (MRONJ). Br J Oral Maxillofac Surg. 2020;58:771-776.
Ruggiero SL, Dodson TB, Aghaloo T, Carlson ER, Ward BB, Kademani D. AAOMS’ position paper on MRONJ-2022 update. J Oral Maxillofac Surg. 2022;80:920-943.
Campisi G, Mauceri R, Bertoldo F, et al. Medication-related osteonecrosis of jaws (MRONJ) prevention and diagnosis: Italian consensus update 2020. Int J Environ Res Public Health. 2020;17:5998.
Lodi G, Sardella A, Salis A, Demarosi F, Tarozzi M, Carrassi A. Tooth extraction in patients taking intravenous bisphosphonates: a preventive protocol and case series. J Oral Maxillofac Surg. 2010;68:107-110.
Saia G, Blandamura S, Bettini G, et al. Occurrence of bisphosphonate-related osteonecrosis of the jaw after surgical tooth extraction. J Oral Maxillofac Surg. 2010;68:797-804.
Vescovi P, Giovannacci I, Merigo E, et al. Tooth extractions in high- risk patients under bisphosphonate therapy and previously affected with osteonecrosis of the jaws: surgical protocol supported by low-level laser therapy. J Craniofac Surg. 2015;26:696-699.
Ata-Ali J, Ata-Ali F, Peñarrocha-Oltra D, Galindo-Moreno P. What is the impact of bisphosphonate therapy upon dental implant survival? A systematic review and meta-analysis. Clin Oral Impl Res. 2016;27:e38-e46.
Pimolbutr K, Porter S, Fedele S. Osteonecrosis of the jaw associated with antiangiogenics in antiresorptive-naïve patient: a comprehensive review of the literature. Biomed Res Int. 2018;2018:1-14.
Curi MM, Condezo AFB, Ribeiro Kd CB, Cardoso CL. Long-term success of dental implants in patients with head and neck cancer after radiation therapy. Int J Oral Maxillofac Surg. 2018;47:783-788.
Gupta S. Dental implant survival rate in irradiated and non- radiated patients: a systematic review and meta-analysis. J Biol Regul Homeost Agents. 2021;35:53-65.
Batstone M. Reconstruction of major defects of the jaws. Aust Dent J. 2018;63:S108-S113.
Ihde S, Kopp S, Gundlach K, Konstantinović VS. Effects of radiation therapy on craniofacial and dental implants: a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:56-65.
Georgiou KR, Hui SK, Xian CJ. Regulatory pathways associated with bone loss and bone marrow adiposity caused by aging, chemotherapy, glucocorticoid therapy and radiotherapy. Am J Stem Cells. 2012;1:205-224.
Costa S, Reagan MR. Therapeutic irradiation: consequences for bone and bone marrow adipose tissue. Front Endocrinol. 2019;10:587.
Schiegnitz E, Müller LK, Sagheb K, et al. Clinical long-term and patient-reported outcomes of dental implants in oral cancer patients. Int J Implant Dent. 2021;7:93.
Shih A, Miaskowski C, Dodd MJ, Stotts NA, MacPhail L. Mechanisms for radiation-induced oral mucositis and the consequences. Cancer Nurs. 2003;26:222-229.
Silverman S. Oral cancer: complications of therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:122-126.
Shokouhi B, Cerajewska T. Radiotherapy and the survival of dental implants: a systematic review. Br J Oral Maxillofac Surg. 2022;60:422-429.
Doll C, Nack C, Raguse JD, et al. Survival analysis of dental implants and implant-retained prostheses in oral cancer patients up to 20 years. Clin Oral Invest. 2015;19:1347-1352.
Sammartino G, Marenzi G, Cioffi I, Teté S, Mortellaro C. Implant therapy in irradiated patients. J Craniofac Surg. 2011;22:443-445.
Javed F, Al-Hezaimi K, Al-Rasheed A, Almas K, Romanos GE. Implant survival rate after oral cancer therapy: a review. Oral Oncol. 2010;46:854-859.
Verdonck HWD, de Jong JMA, Granzier MEPG, Nieman FH, de Baat C, Stoelinga PJW. Intensity-modulated radiation therapy for oropharyngeal cancer: radiation dosage constraint at the anterior mandible. Oral Oncol. 2009;45:511-514.
Nooh N. Dental implant survival in irradiated oral cancer patients: a systematic review of the literature. Int J Oral Maxillofac Implants. 2013;28:1233-1242.
Shugaa-Addin B, Al-Shamiri H, Al-Maweri S, Tarakji B. The effect of radiotherapy on survival of dental implants in head and neck cancer patients. J Clin Exp Dent. 2016;8:e194-e200.
Asikainen P, Klemetti E, Kotilainen R, et al. Osseointegration of dental implants in bone irradiated with 40, 50 or 60 Gy doses. An experimental study with beagle dogs: titanium implants and irradiated bone. Clin Oral Implants Res. 1998;9:20-25.
Lee J, Lee JJB, Cha I, Park KR, Lee CG. Risk factor analysis of dental implants in patients with irradiated head and neck cancer. Head Neck. 2022;44:1816-1824.
Schoen PJ, Reintsema H, Raghoebar GM, Vissink A, Roodenburg JLN. The use of implant retained mandibular prostheses in the oral rehabilitation of head and neck cancer patients. A review and rationale for treatment planning. Oral Oncol. 2004;40:862-871.
Esposito M, Hirsch JM, Lekholm U, Thomsen P. Biological factors contributing to failures of osseointegrated oral implants, (II). Etiopathogenesis: biological factors contributing to failures of osseointegrated oral implants, (II). Etiopathogenesis. Eur J Oral Sci. 1998;106:721-764.
Visch LL, van Waas MAJ, Schmitz PIM, Levendag PC. A clinical evaluation of implants in irradiated oral cancer patients. J Dent Res. 2002;81:856-859.
Yerit KC, Posch M, Seemann M, et al. Implant survival in mandibles of irradiated oral cancer patients. Clin Oral Implants Res. 2006;17:337-344.
Claudy MP, Miguens SAQ, Celeste RK, Camara Parente R, Hernandez PAG, da Silva AN. Time interval after radiotherapy and dental implant failure: systematic review of observational studies and meta-analysis: radiotherapy and dental implant failure. Clin Implant Dent Relat Res. 2015;17:402-411.
Schliephake H, Neukam FW, Schmelzeisen R, Wichmann M. Long-term results of endosteal implants used for restoration of oral function after oncologic surgery. Int J Oral Maxillofac Surg. 1999;28:260-265.
Brogniez V, Lejuste P, Pecheur A, Reychler H. Dental prosthetic reconstruction of osseointegrated implants placed in irradiated bone. Int J Oral Maxillofac Implants. 1998;13:506-512.
Hancock PJ, Epstein JB, Sadler GR. Oral and dental management related to radiation therapy for head and neck cancer. J Can Dent Assoc. 2003;69:585-590.
Kovacs AF. The fate of osseointegrated implants in patients following oral cancer surgery and mandibular reconstruction. Head Neck. 2000;22:111-119.
Taylor TD, Worthington P. Osseointegrated implant rehabilitation of the previously irradiated mandible: results of a limited trial at 3 to 7 years. J Prosthet Dent. 1993;69:60-69.
Anderson L, Meraw S, Al-Hezaimi K, Wang HL. The influence of radiation therapy on dental Implantology. Implant Dent. 2013;22:31-38.
Mian TA, Van Putten MC, Kramer DC, Jacob RF, Boyer AL. Backscatter radiation at bone-titanium interface from high-energy x and gamma rays. Int J Radiat Oncol Biol Phys. 1987;13:1943-1947.
Harpaz R, Dahl RM, Dooling KL. Prevalence of immunosuppression among US adults, 2013. JAMA. 2016;316:2547-2548.
Duttenhoefer F, Fuessinger MA, Beckmann Y, Schmelzeisen R, Groetz KA, Boeker M. Dental implants in immunocompromised patients: a systematic review and meta-analysis. Int J Implant Dent. 2019;5:43.
Calder JDF, Buttery L, Revell PA, Pearse M, Polak JM. Apoptosis – a significant cause of bone cell death in osteonecrosis of the femoral head. J Bone Joint Surg Br. 2004;86:1209-1213.
Mitra R. Adverse effects of corticosteroids on bone metabolism: a review. PM R. 2011;3:466-471.
Shen EC, Fu E, Hsieh YD. Effects of cyclosporin a on dental alveolar bone: a histomorphometric study in rats. J Periodontol. 2001;72:659-665.
da Silva PF, Pallos D, Silva Queiroz C, Ricardo LH. Previous exposure to cyclosporine a and periodontal breakdown in rats. Arch Oral Biol. 2015;60:566-573.
Fujimoto T, Niimi A, Sawai T, Ueda M. Effects of steroid-induced osteoporosis on osseointegration of titanium implants. Int J Oral Maxillofac Implants. 1998;13:183-189.
Petsinis V, Kamperos G, Alexandridi F, Alexandridis K. The impact of glucocorticosteroids administered for systemic diseases on the osseointegration and survival of dental implants placed without bone grafting – a retrospective study in 31 patients. J Craniomaxillofac Surg. 2017;45:1197-1200.
Thomason JM, Girdler NM, Kendall-Taylor P, Wastell H, Weddel A, Seymour RA. An investigation into the need for supplementary steroids in organ transplant patients undergoing gingival surgery: a double-blind, split-mouth, cross-over study. J Clin Periodontol. 1999;26:577-582.
Gibson N, Ferguson JW. Steroid cover for dental patients on long-term steroid medication: proposed clinical guidelines based upon a critical review of the literature. Br Dent J. 2004;197: 681-685.
Burtscher D, Dalla TD. Dental implant procedures in immunosuppressed organ transplant patients: a systematic review. Int J Oral Maxillofac Surg. 2022;51:380-387.
Weiss RA. How does HIV cause AIDS? Science. 1993;260(5112):1273-1279.
Ata-Ali J, Ata-Ali F, Di-Benedetto N, Bagán L, Bagán JV. Does HIV infection have an impact upon dental implant osseointegration? A systematic review. Med Oral Patol Oral Cir Bucal. 2015;20:e347-e356.
Castronuovo D, Cacopardo B, Pinzone MR, et al. Bone disease in the setting of HIV infection: update and review of the literature. Eur Rev Med Pharmacol Sci. 2013;17:2413-2419.
Dolan SE, Huang JS, Killilea KM, Sullivan MP, Aliabadi N, Grinspoon S. Reduced bone density in HIV-infected women. AIDS. 2004;18:475-483.
Yin MT, Modarresi R, Shane E, et al. Effects of HIV infection and antiretroviral therapy with ritonavir on induction of osteoclastlike cells in postmenopausal women. Osteoporos Int. 2011;22:1459-1468.
Fakruddin JM, Laurence J. HIV envelope gp120-mediated regulation of Osteoclastogenesis via receptor activator of nuclear factor κB ligand (RANKL) secretion and its modulation by certain HIV protease inhibitors through interferon-γ/RANKL cross-talk. J Biol Chem. 2003;278:48251-48258.
Fakruddin JM, Laurence J. Interactions among human immunodeficiency virus (HIV)-1, interferon- γ and receptor of activated NF-κ B ligand (RANKL): implications for HIV pathogenesis. Clin Exp Immunol. 2004;137:538-545.
May MC, Andrews PN, Daher S, Reebye UN. Prospective cohort study of dental implant success rate in patients with AIDS. Int J Implant Dent. 2016;2:20.
Glick M, Abel SN, Muzyka BC, Delorenzo M. Dental complications after treating patients with Aids. J Am Dent Assoc. 1994;125:296-301.
Patton LL, Shugars DA, Bonito AJ. A systematic review of complication risks for HIV-positive patients undergoing invasive dental procedures. J Am Dent Assoc. 2002;133:195-203.
Neumeier TT, Reddy M, Geurs N, Hill J, Neumeier H. Longitudinal study of dental implants in HIV−positive patients. J Prosthodont. 2022;31:115-120.
Vidal F, Peres RV, Souza RC, Gonçalves C, Pavan P, Gonçalves LS. Dental implants in individuals living with HIV-1: results from a prospective study in patients undergoing highly active antiretroviral therapy. Spec Care in Dentist. 2022;42:112-119.
Sivakumar I, Arunachalam S, Choudhary S, Buzayan MM. Does HIV infection affect the survival of dental implants? A systematic review and meta-analysis. J Prosthet Dent. 2021;125:862-869.
Lemos CAA, Verri FR, Cruz RS, Santiago Júnior JF, Faverani LP, Pellizzer EP. Survival of dental implants placed in HIV-positive patients: a systematic review. Int J Oral Maxillofac Surg. 2018;47:1336-1342.
Casula L, Poli A, Clemente T, Artuso G, Capparé P, Gherlone EF. Prevalence of peri-implantitis in a sample of HIV-positive patients. Clin Exp Dent Res. 2021;7:1002-1013.
Gay-Escoda C, Perez-Alvarez D, Camps-Font O, Figueiredo R. Long-term outcomes of oral rehabilitation with dental implants in HIV-positive patients: a retrospective case series. Med Oral. 2016;21:e385-e391.
Sabbah A, Hicks J, MacNeill B, et al. A retrospective analysis of dental implant survival in HIV patients. J Clin Periodontol. 2019;46:363-372.
de Oliveira MA, Pallos D, Mecca F, et al. Dental implants in patients seropositive for HIV. J Am Dent Assoc. 2020;151:863-869.
Pikner SS, Gröndahl K. Radiographic analyses of “advanced” marginal bone loss around Brånemark® dental implants. Clin Implant Dent Relat Res. 2009;11:120-133.
Mahy M, Marsh K, Sabin K, Wanyeki I, Daher J, Ghys PD. HIV estimates through 2018: data for decision-making. AIDS. 2019;33:S203-S211.
Shirlaw P, Chikte U, MacPhail L, Schmidt-Westhausen A, Croser D, Reichart P. Oral and dental care and treatment protocols for the management of HIV-infected patients: oral care and treatment protocols in HIV. Oral Dis. 2002;8:136-143.
Zam SNA, Sylvyana M, Sjamsudin E. Management of third molar surgery in HIV-positive patients. Oral Dis. 2020;26:145-148.
Nabel EG. Cardiovascular disease. N Engl J Med. 2003;349:60-72.
Khadivi V, Anderson J, Zarb GA. Cardiovascular disease and treatment outcomes with osseointegration surgery. J Prosthet Dent. 1999;81:533-536.
Neves J, de Araújo NM, Oliveira P, Martins dos Santos J, Malo P. Risk factors for implant failure and peri-implant pathology in systemic compromised patients: outcome predictors in the systemically compromised. J Prosthodont. 2018;27:409-415.
Niedermaier R, Stelzle F, Riemann M, Bolz W, Schuh P, Wachtel H. Implant-supported immediately loaded fixed full-arch dentures: evaluation of implant survival rates in a case cohort of up to 7 years: immediately loaded fixed full-arch dentures. Clin Implant Dent Relat Res. 2017;19:4-19.
Nobre M d A, Maló P, Gonçalves Y, Sabas A, Salvado F. Outcome of dental implants in diabetic patients with and without cardiovascular disease: a 5-year post-loading retrospective study. Eur J Oral Implantol. 2016;9:87-95.
Wu X, Al-Abedalla K, Eimar H, et al. Antihypertensive medications and the survival rate of osseointegrated dental implants: a cohort study: antihypertensive drugs and dental implants. Clin Implant Dent Relat Res. 2016;18:1171-1182.
Hasanoglu Erbasar GN, Hocaoğlu TP, Erbasar RC. Risk factors associated with short dental implant success: a long-term retrospective evaluation of patients followed up for up to 9 years. Braz Oral Res. 2019;33:e030.
Mayta-Tovalino F, Mendoza-Martiarena Y, Romero-Tapia P, et al. An 11-year retrospective research study of the predictive factors of peri-implantitis and implant failure: analytic-multicentric study of 1279 implants in Peru. Int J Dent. 2019;2019:1-8.
Nguyen TTH, Eo MY, Cho YJ, Myoung H, Kim SM. 7-mm-long dental implants: retrospective clinical outcomes in medically compromised patients. J Korean Assoc Oral Maxillofac Surg. 2019;45:260.
Schimmel M, Srinivasan M, McKenna G, Müller F. Effect of advanced age and/or systemic medical conditions on dental implant survival: a systematic review and meta-analysis. Clin Oral Impl Res. 2018;29:311-330.
Froum S, Hengjeerajaras P, Liu KY, Maketone P, Patel V, Shi Y. The link between periodontitis/peri-implantitis and cardiovascular disease: a systematic literature review. Int J Periodontics Restorative Dent. 2020;40:e229-e233.
Hwang D, Wang HL. Medical contraindications to implant therapy: part I: absolute contraindications. Implant Dent. 2006;15:353-360.
Diz P, Scully C, Sanz M. Dental implants in the medically compromised patient. J Dent. 2013;41:195-206.
Jolly DE. Evaluation of the medical history. Anesth Prog. 1995;42:84-89.
Kämmerer PW, Frerich B, Liese J, Schiegnitz E, Al-Nawas B. Oral surgery during therapy with anticoagulants – a systematic review. Clin Oral Invest. 2015;19:171-180.
Dawoud BES, Kent S, Tabbenor O, George P, Dhanda J. Dental implants and risk of bleeding in patients on oral anticoagulants: a systematic review and meta-analysis. Int J Implant Dent. 2021;7:82.
Miziara LNB, Sendyk WR, Ortega KL, Gallottini M, Sendyk DI, Martins F. Risk of bleeding during implant surgery in patients taking antithrombotics: a systematic review. Semin Thromb Hemost. 2021;47:702-708.
Malik A, Majeed S. Effect of antiplatelet therapy on minor dental procedures. Natl J Maxillofac Surg. 2020;11:64-66.
Iwabuchi H, Imai Y, Asanami S, et al. Evaluation of postextraction bleeding incidence to compare patients receiving and not receiving warfarin therapy: a cross-sectional, multicentre, observational study. BMJ Open. 2014;4:e005777.
Airoldi G, Campanini M. Apixaban. Apixaban it J Med. 2011;5:128-134.
Napeñas JJ, Patton LL. Bleeding and clotting disorders. In: Glick M, Greenberg MS, Lockhart PB, Challacombe SJ, eds. Burket’s Oral Medicine. 1st ed. Wiley; 2021:665-704.
Douketis J, Tosetto A, Marcucci M, et al. Risk of recurrence after venous thromboembolism in men and women: patient level meta- analysis. BMJ. 2011;342:d813.
Yong JW, Yang LX, Ohene BE, Zhou YJ, Wang ZJ. Periprocedural heparin bridging in patients receiving oral anticoagulation: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2017;17:295.
Römer P, Heimes D, Pabst A, Becker P, Thiem DGE, Kämmerer PW. Bleeding disorders in implant dentistry: a narrative review and a treatment guide. Int J Implant Dent. 2022;8:20.
Morse RM. The definition of alcoholism. JAMA. 1992;268:1012.
Ouanounou A, Hassanpour S, Glogauer M. The influence of systemic medications on osseointegration of dental implants. J Can Dent Assoc. 2016;82:g7.
Istituto Nazionale per gli Alimenti e la Nutrizione. Linee guida per una sana alimentazione italiana. Ministero delle Politiche Agricole e Forestali, INRAN; 2003.
Baran DT, Teitelbaum SL, Bergfeld MA, Parker G, Cruvant EM, Avioli LV. Effect of alcohol ingestion on bone and mineral metabolism in rats. Am J Physiol. 1980;238:E507-E510.
Turner RT, Aloia RC, Segel LD, Hannon KS, Bell NH. Chronic alcohol treatment results in disturbed vitamin D metabolism and skeletal abnormalities in rats. Alcohol Clin Exp Res. 1988;12:159-162.
Friday KE, Howard GA. Ethanol inhibits human bone cell proliferation and function in vitro. Metabolism. 1991;40:562-565.
Farley JR, Fitzsimmons R, Taylor AK, Jorch UM, Lau KHW. Direct effects of ethanol on bone resorption and formation in vitro. Arch Biochem Biophys. 1985;238:305-314.
Koo S, König B, Mizusaki CI, Allegrini S, Yoshimoto M, Carbonari MJ. Effects of alcohol consumption on osseointegration of titanium implants in rabbits. Implant Dent. 2004;13:232-237.
Diamond T, Stiel D, Lunzer M, Wilkinson M, Posen S. Ethanol reduces bone formation and may cause osteoporosis. Am J Med. 1989;86:282-288.
Taïeb J, Delarche C, Ethuin F, et al. Ethanol-induced inhibition of cytokine release and protein degranulation in human neutrophils. J Leukoc Biol. 2002;72:1142-1147.
Lockhart PB, Gibson J, Pond SH, Leitch J. Dental management considerations for the patient with an acquired coagulopathy. Part 1: coagulopathies from systemic disease. Br Dent J. 2003;195:439-445.
Block MS, Christensen BJ, Mercante DE, Chapple AG. What factors are associated with implant failure? J Oral Maxillofac Surg. 2021;79:91-97.
Alissa R, Oliver RJ. Influence of prognostic risk indicators on osseointegrated dental implant failure: a matched case-control analysis. J Oral Implantol. 2012;38:51-61.
Carnelio S, Khan SA, Rodrigues G. Definite, probable or dubious: antioxidants trilogy in clinical dentistry. Br Dent J. 2008;204:29-32.
Kopáni M, Celec P, Danišovič L, Michalka P, Biró C. Oxidative stress and electron spin resonance. Clin Chim Acta. 2006;364:61-66.
Pua ML, Yoshitomi T, Chonpathompikunlert P, Hirayama A, Nagasaki Y. Redox-active injectable gel using thermo-responsive nanoscale polyion complex flower micelle for noninvasive treatment of local inflammation. J Control Release. 2013;172:914-920.
Yanez M, Blanchette J, Jabbarzadeh E. Modulation of inflammatory response to implanted biomaterials using natural compounds. Curr Pharm des. 2018;23:6347-6357.
Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4:118-126.
Aksakalli S. Antioxidants in dentistry: review of literature. Dentistry. 2013;4:1.
Knaś M, Maciejczk M, Waszkiel D, Zalewska A. Oxidative stress and salivary antioxidants. Dent Med Probl. 2013;50:461-466.
Esterbauer H, Puhl H, Dieber-rotheneder M, Waeg G, Rabl H. Effect of antioxidants on oxidative modification of LDL. Ann Med. 1991;23:573-581.
Tóthová L, Celec P. Oxidative stress and antioxidants in the diagnosis and therapy of periodontitis. Front Physiol. 2017;8:1055.
TAAo P. Parameter on chronic periodontitis with slight to moderate loss of periodontal support. J Periodontol. 2000;71:853-855.
Kornman KS. Mapping the pathogenesis of periodontitis: a new look. J Periodontol. 2008;79:1560-1568.
Muniz FWMG, Nogueira SB, Mendes FLV, et al. The impact of antioxidant agents complimentary to periodontal therapy on oxidative stress and periodontal outcomes: a systematic review. Arch Oral Biol. 2015;60:1203-1214.
Tsaryk R, Kalbacova M, Hempel U, et al. Response of human endothelial cells to oxidative stress on Ti6Al4V alloy. Biomaterials. 2007;28:806-813.
Lee MC, Yoshino F, Shoji H, et al. Characterization by electron spin resonance spectroscopy of reactive oxygen species generated by titanium dioxide and hydrogen peroxide. J Dent Res. 2005;84:178-182.
Bressan E, Ferroni L, Gardin C, et al. Metal nanoparticles released from dental implant surfaces: potential contribution to chronic inflammation and peri-implant bone loss. Materials. 2019;12:2036.
Delgado-Ruiz R, Romanos G. Potential causes of titanium particle and ion release in implant dentistry: a systematic review. Int J Mol Sci. 2018;19:3585.
Abdulhameed EA, Al-Rawi NH, Omar M, Khalifa N, Samsudin ABR. Titanium dioxide dental implants surfaces related oxidative stress in bone remodeling: a systematic review. PeerJ. 2022;10:e12951.
Caton JG, Armitage G, Berglundh T, et al. A new classification scheme for periodontal and peri-implant diseases and conditions – introduction and key changes from the 1999 classification. J Periodontol. 2018;89:S1-S8.
Esquivel-Chirino C, Gómez-Landeros JC, Carabantes-Campos EP, et al. The impact of oxidative stress on dental implants. EJDENT. 2021;2:1-8.
Mousavi Jazi M, Sadeghi Pour Rodsari HR, Mirmiran F. Level of oxidative stress markers in Peri-implant crevicular fluid and their correlation with clinical parameters. J Dent. 2015;12:340-346.
Mouthuy PA, Snelling SJB, Dakin SG, et al. Biocompatibility of implantable materials: an oxidative stress viewpoint. Biomaterials. 2016;109:55-68.
Kaur G, Kathariya R, Bansal S, Singh A, Shahakar D. Dietary antioxidants and their indispensable role in periodontal health. J Food Drug Anal. 2016;24:239-246.
Tardy BL, Richardson JJ, Nithipipat V, et al. Protein adsorption and coordination-based end-tethering of functional polymers on metal–phenolic network films. Biomacromolecules. 2019;20:1421-1428.
Harmankaya N, Igawa K, Stenlund P, Palmquist A, Tengvall P. Healing of complement activating Ti implants compared with non-activating Ti in rat tibia. Acta Biomater. 2012;8:3532-3540.
Kalltorp M, Askendal A, Thomsen P, Tengvall P. Inflammatory cell recruitment, distribution, and chemiluminescence response at IgG precoated – and thiol functionalized gold surfaces. J Biomed Mater Res. 1999;47:251-259.
Di H, Qiaoxia L, Yujie Z, et al. Ag nanoparticles incorporated tannic acid/nanoapatite composite coating on Ti implant surfaces for enhancement of antibacterial and antioxidant properties. Surf Coat Technol. 2020;399:126169.
Catauro M, Papale F, Bollino F, et al. Silica/quercetin sol–gel hybrids as antioxidant dental implant materials. Sci Technol Adv Mater. 2015;16:035001.
Catauro M, Bollino F, Papale F, Ferrara C, Mustarelli P. Silica– polyethylene glycol hybrids synthesized by sol–gel: biocompatibility improvement of titanium implants by coating. Mater Sci Eng C Mater Biol Appl. 2015;55:118-125.
Rathee P, Chaudhary H, Rathee S, Rathee D, Kumar V, Kohli K. Mechanism of action of flavonoids as anti-inflammatory agents: a review. Inflamm Allergy Drug Targets. 2009;8:229-235.
Catauro M, Bollino F, Papale F, Piccolella S, Pacifico S. Sol–gel synthesis and characterization of SiO2/PCL hybrid materials containing quercetin as new materials for antioxidant implants. Mater Sci Eng C Mater Biol Appl. 2016;58:945-952.
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231-1237.
Graham HD. Stabilization of the Prussian blue color in the determination of polyphenols. J Agric Food Chem. 1992;40:801-805.
Boraldi F, Annovi G, Paolinelli-Devincenzi C, Tiozzo R, Quaglino D. The effect of serum withdrawal on the protein profile of quiescent human dermal fibroblasts in primary cell culture. Proteomics. 2008;8:66-82.
Chow CK, Ibrahim W, Wei Z, Chan AC. Vitamin E regulates mitochondrial hydrogen peroxide generation. Free Radic Biol Med. 1999;27:580-587.
Costa L, Carpentieri I, Bracco P. Post electron-beam irradiation oxidation of orthopaedic ultra-high molecular weight polyethylene (UHMWPE) stabilized with vitamin E. Polym Degrad Stab. 2009;94:1542-1547.
Ozawa R, Saita M, Sakaue S, et al. Redox injectable gel protects osteoblastic function against oxidative stress and suppresses alveolar bone loss in a rat peri-implantitis model. Acta Biomater. 2020;110:82-94.
Qi F, Huang H, Wang M, Rong W, Wang J. Applications of antioxidants in dental procedures. Antioxidants. 2022;11:2492.
Stevens JL, Feelisch M, Martin DS. Perioperative oxidative stress: the unseen enemy. Anesth Analg. 2019;129:1749-1760.
Chang D, Wang F, Zhao YS, Pan HZ. Evaluation of oxidative stress in colorectal cancer patients. Biomed Environ Sci. 2008;21:286-289.
Leufkens AM, van Duijnhoven FJB, Woudt SHS, et al. Biomarkers of oxidative stress and risk of developing colorectal cancer: a cohortnested case-control study in the European Prospective Investigation into Cancer and Nutrition. Am J Epidemiol. 2012;175:653-663.
Zińczuk M, Zaręba R, Markowski K, et al. Antioxidant barrier, redox status, and oxidative damage to biomolecules in patients with colorectal cancer. Can malondialdehyde and catalase be markers of colorectal cancer advancement? Biomolecules. 2019;9:637.
Chiang FF, Chao TH, Huang SC, Cheng CH, Tseng YY, Huang YC. Cysteine regulates oxidative stress and glutathione-related Antioxidative capacity before and after colorectal tumor resection. Int J Mol Sci. 2022;23:9581.
